CIE AS/A Level Chemistry 30.1 Arenes Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 30.1 Arenes Study Notes – New Syllabus
CIE AS/A Level Chemistry 30.1 Arenes Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- describe the chemistry of arenes as exemplified by the following reactions of benzene and methylbenzene:
(a) substitution reactions with Cl₂ and with Br₂ in the presence of a catalyst, AlCl₃ or AlBr₃, to form halogenoarenes (aryl halides)
(b) nitration with a mixture of concentrated HNO₃ and concentrated H₂SO₄ at a temperature between 25°C and 60°C
(c) Friedel–Crafts alkylation by CH₃Cl and AlCl₃ and heat
(d) Friedel–Crafts acylation by CH₃COCl and AlCl₃ and heat
(e) complete oxidation of the side-chain using hot alkaline KMnO₄ and then dilute acid to give a benzoic acid
(f) hydrogenation of the benzene ring using H₂ and Pt/Ni catalyst and heat to form a cyclohexane ring - describe the mechanism of electrophilic substitution in arenes:
(a) as exemplified by the formation of nitrobenzene and bromobenzene
(b) with regards to the effect of delocalisation (aromatic stabilisation) of electrons in arenes to explain thepredomination of substitution over addition - predict whether halogenation will occur in the side-chain or in the aromatic ring in arenes depending on reaction conditions
- describe that in the electrophilic substitution of arenes, different substituents direct to different ring positions (limited to the directing effects of –NH₂, –OH, –R, –NO₂, –COOH and –COR)
Chemistry of Arenes: Reactions of Benzene and Methylbenzene
Arenes such as benzene and methylbenzene undergo characteristic reactions that preserve, or in specific cases destroy, the aromatic ring. You must be able to describe and explain the chemistry of arenes using the reactions listed below.
General Reaction Type
Most reactions of benzene and methylbenzene are electrophilic substitution reactions.
Substitution occurs rather than addition because addition would disrupt the delocalised π system and reduce aromatic stability.
(a) Halogenation with Cl₂ or Br₂
Benzene and methylbenzene react with chlorine or bromine in the presence of a halogen carrier catalyst, AlCl₃ or AlBr₃.

The catalyst polarises the halogen molecule to generate a strong electrophile.
Example:
\( \mathrm{C_6H_6 + Cl_2 \xrightarrow{AlCl_3} C_6H_5Cl + HCl} \)
The product is a halogenoarene (aryl halide).
(b) Nitration
Nitration is carried out using a mixture of concentrated nitric acid and concentrated sulfuric acid at 25–60°C.
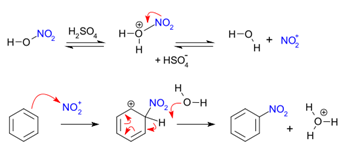
Sulfuric acid generates the nitronium ion, \( \mathrm{NO_2^+} \), which acts as the electrophile.
\( \mathrm{C_6H_6 + HNO_3 \xrightarrow{H_2SO_4} C_6H_5NO_2 + H_2O} \)
Methylbenzene reacts faster than benzene because the methyl group donates electron density to the ring.
(c) Friedel–Crafts Alkylation
Friedel–Crafts alkylation introduces an alkyl group onto the benzene ring.
Conditions: chloroalkane (e.g. CH₃Cl), AlCl₃ catalyst and heat.
\( \mathrm{C_6H_6 + CH_3Cl \xrightarrow{AlCl_3} C_6H_5CH_3 + HCl} \)
This reaction proceeds via an electrophilic substitution mechanism.
(d) Friedel–Crafts Acylation
Friedel–Crafts acylation introduces an acyl group onto the benzene ring using an acyl chloride.
Conditions: CH₃COCl, AlCl₃ catalyst and heat.
\( \mathrm{C_6H_6 + CH_3COCl \xrightarrow{AlCl_3} C_6H_5COCH_3 + HCl} \)
The product is a phenyl ketone. Acylation occurs once because the acyl group deactivates the ring.
(e) Oxidation of the Side-Chain
Alkyl side-chains on benzene rings can be oxidised completely to a carboxylic acid.

Conditions: hot alkaline \( \mathrm{KMnO_4} \), followed by acidification.
Methylbenzene is oxidised to benzoic acid:
\( \mathrm{C_6H_5CH_3 \rightarrow C_6H_5COOH} \)
The benzene ring itself remains unchanged.
(f) Hydrogenation of the Benzene Ring
Under forcing conditions, the benzene ring can be hydrogenated.
Conditions: hydrogen gas, Pt or Ni catalyst and heat.
\( \mathrm{C_6H_6 + 3H_2 \xrightarrow{Pt/Ni} C_6H_{12}} \)
This reaction destroys the delocalised π system and forms cyclohexane.
Example
Explain why benzene reacts with bromine only in the presence of AlBr₃.
▶️ Answer / Explanation
Benzene is stabilised by a delocalised π system and is resistant to reaction.
AlBr₃ acts as a catalyst to generate a strong electrophile from bromine.
This allows electrophilic substitution to occur without destroying aromaticity.
Example
Describe the reaction that converts methylbenzene into benzoic acid and explain why the benzene ring is unchanged.
▶️ Answer / Explanation
Methylbenzene is heated with alkaline potassium manganate(VII), causing complete oxidation of the side-chain.
The product is benzoic acid after acidification.
The benzene ring is unchanged because the delocalised π system is stable and resistant to oxidation.
Mechanism of Electrophilic Substitution in Arenes
Arenes such as benzene undergo electrophilic substitution reactions. You must be able to describe the mechanism, using the examples of nitration and bromination, and explain why substitution predominates over addition due to electron delocalisation.

Overview of Electrophilic Substitution
In electrophilic substitution, an electrophile attacks the benzene ring, temporarily disrupting aromaticity, before a hydrogen atom is removed to restore the delocalised π system.
Key Stages of the Mechanism
- generation of a strong electrophile
- attack on the π electrons of the benzene ring to form a sigma complex (arenium ion)
- loss of a proton to regenerate the delocalised π system
(a) Nitration of Benzene → Nitrobenzene
Nitration uses a mixture of concentrated nitric acid and concentrated sulfuric acid.
Sulfuric acid generates the electrophile, the nitronium ion:
\( \mathrm{HNO_3 + H_2SO_4 \rightarrow NO_2^+ + HSO_4^- + H_2O} \)
Step 1: Electrophilic attack
The nitronium ion is attracted to the delocalised π electrons of benzene. A π bond donates a pair of electrons to the electrophile, forming a sigma complex.
Step 2: Deprotonation
The hydrogen atom is removed by \( \mathrm{HSO_4^-} \), restoring the delocalised π system and forming nitrobenzene.
(a) Bromination of Benzene → Bromobenzene
Bromination requires a halogen carrier catalyst, such as AlBr₃.
The catalyst polarises bromine to generate an electrophile:
\( \mathrm{Br_2 + AlBr_3 \rightarrow Br^+ + AlBr_4^-} \)
Step 1: Electrophilic attack
The \( \mathrm{Br^+} \) ion attacks the π system of benzene, forming a sigma complex.
Step 2: Deprotonation
A proton is removed by \( \mathrm{AlBr_4^-} \), regenerating the delocalised π system and forming bromobenzene.
(b) Role of Delocalisation: Substitution vs Addition
Benzene is stabilised by a delocalised π electron system spread over all six carbon atoms.
During electrophilic substitution:
- delocalisation is temporarily disrupted in the sigma complex
- it is restored when a proton is removed
In an addition reaction, the delocalised π system would be permanently destroyed, producing a much less stable molecule.
Therefore, substitution is favoured over addition because it preserves aromatic stabilisation.
Example
Describe the role of the nitronium ion in the nitration of benzene.
▶️ Answer / Explanation
The nitronium ion acts as the electrophile.
It accepts a pair of electrons from the delocalised π system of benzene to form a sigma complex.
Loss of a proton then restores the delocalised π system.
Example
Explain why benzene undergoes electrophilic substitution rather than electrophilic addition.
▶️ Answer / Explanation
Benzene contains a delocalised π electron system that provides aromatic stabilisation.
In substitution reactions, this delocalisation is only temporarily disrupted and then restored.
In addition reactions, the delocalised π system would be destroyed permanently, making the product much less stable.
Therefore, substitution is favoured over addition.
Halogenation of Arenes: Ring vs Side-Chain
Alkyl-substituted arenes, such as methylbenzene, can undergo halogenation in two different locations. You must be able to predict whether halogenation occurs in the aromatic ring or in the side-chain, depending on the reaction conditions.
Halogenation in the Aromatic Ring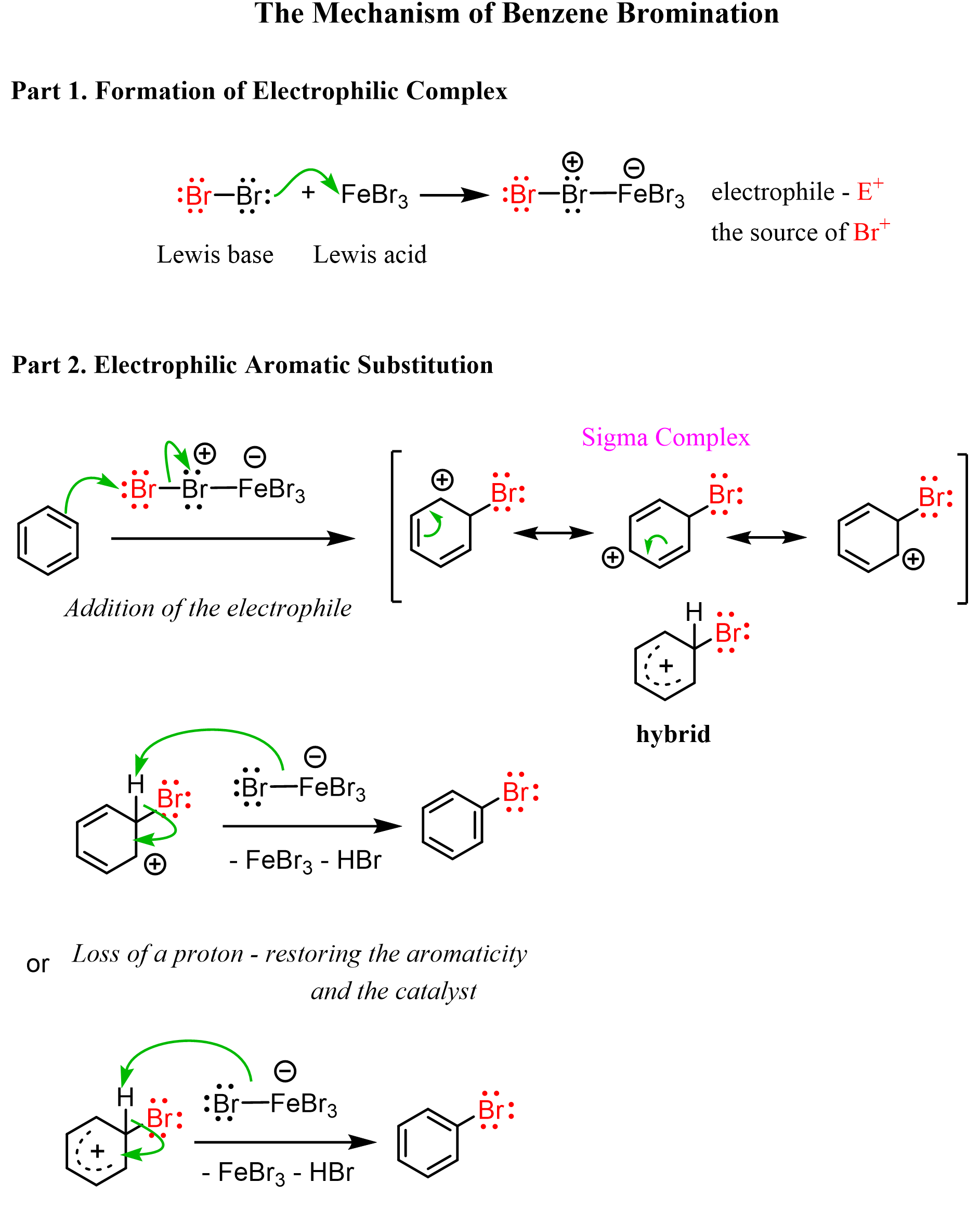
Halogenation occurs in the benzene ring under conditions that favour electrophilic substitution.
Conditions:
- \( \mathrm{Cl_2} \) or \( \mathrm{Br_2} \)
- halogen carrier catalyst (AlCl₃ or AlBr₃)
- room temperature
- absence of UV light
Under these conditions, the halogen acts as an electrophile and replaces a hydrogen atom on the ring.
Example:
\( \mathrm{C_6H_5CH_3 + Br_2 \xrightarrow{AlBr_3} BrC_6H_4CH_3 + HBr} \)
This is an electrophilic substitution reaction.
Halogenation in the Side-Chain
Halogenation occurs in the alkyl side-chain under conditions that favour free-radical substitution.
Conditions:
- \( \mathrm{Cl_2} \) or \( \mathrm{Br_2} \)

- UV light or high temperature
- no halogen carrier catalyst
Under these conditions, a hydrogen atom on the alkyl side-chain is replaced by a halogen atom.
Example:
\( \mathrm{C_6H_5CH_3 + Cl_2 \xrightarrow{UV} C_6H_5CH_2Cl + HCl} \)
This is a free-radical substitution reaction.
Why Conditions Matter
The benzene ring is stabilised by a delocalised π system and only reacts when a strong electrophile is generated by a catalyst.
UV light provides energy to break the halogen–halogen bond homolytically, forming radicals that react preferentially with the alkyl side-chain.
Exam Rule to Remember
Catalyst → ring substitution
UV light → side-chain substitution
Example
Predict where halogenation occurs when methylbenzene reacts with bromine in the presence of AlBr₃.
▶️ Answer / Explanation
Halogenation occurs in the aromatic ring.
AlBr₃ generates a strong electrophile, so the reaction proceeds by electrophilic substitution.
Example
Methylbenzene reacts with chlorine under UV light. Explain why the side-chain is substituted rather than the benzene ring.
▶️ Answer / Explanation
UV light causes homolytic fission of the chlorine–chlorine bond, forming chlorine radicals.
These radicals react by free-radical substitution at the alkyl side-chain.
No halogen carrier catalyst is present, so a strong electrophile is not formed and ring substitution does not occur.
Directing Effects in Electrophilic Substitution of Arenes
In electrophilic substitution reactions of arenes, any substituent already attached to the benzene ring affects both the rate of reaction and the position at which a new substituent is introduced. You must be able to describe how different substituents direct substitution to different ring positions.
Ortho, Meta and Para Positions
Relative to an existing substituent on the benzene ring:
- ortho positions are adjacent to the substituent (1,2-)
- meta positions are separated by one carbon (1,3-)
- para positions are opposite the substituent (1,4-)
Electron-Donating Substituents: Ortho/Para Directors
Substituents that donate electron density to the benzene ring activate the ring and direct substitution to the ortho and para positions.
For this specification, the following are ortho/para-directing groups:
- –NH₂
- –OH
- –R (alkyl group, e.g. –CH₃)
These groups increase electron density in the ring, particularly at the ortho and para positions, making electrophilic attack more likely at these sites.
Electron-Withdrawing Substituents: Meta Directors
Substituents that withdraw electron density from the benzene ring deactivate the ring and direct substitution to the meta position.
For this specification, the following are meta-directing groups:
- –NO₂
- –COOH
- –COR
These groups reduce electron density at the ortho and para positions, so electrophilic substitution occurs preferentially at the meta position.
Summary of Directing Effects
Electron-donating groups → ortho/para directing
Electron-withdrawing groups → meta directing
In practice, a mixture of products may form, but one orientation usually predominates.
Example
Predict the major product when methylbenzene undergoes nitration.
▶️ Answer / Explanation
The –CH₃ group is an electron-donating substituent.
It directs electrophilic substitution to the ortho and para positions.
The major products are ortho- and para-nitromethylbenzene, with the para isomer usually predominating.
Example
Explain why nitration of benzoic acid mainly gives a meta-substituted product.
▶️ Answer / Explanation
The –COOH group is an electron-withdrawing substituent.
It decreases electron density at the ortho and para positions of the benzene ring.
As a result, electrophilic substitution occurs preferentially at the meta position.

