CIE AS/A Level Chemistry 31.1 Halogen compounds Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 31.1 Halogen compounds Study Notes – New Syllabus
CIE AS/A Level Chemistry 31.1 Halogen compounds Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
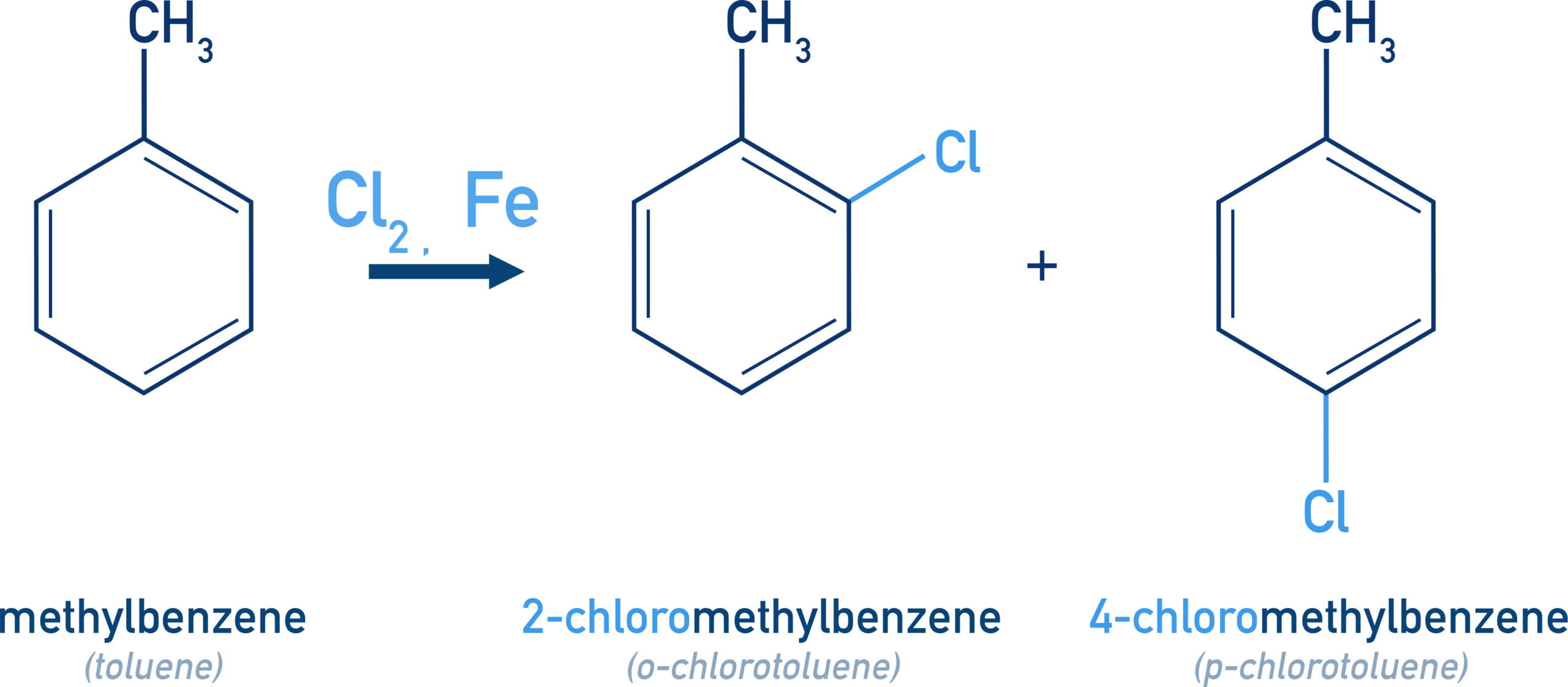
- recall the reactions by which halogenoarenes can be produced: substitution of an arene with Cl₂ or Br₂ in the presence of a catalyst, AlCl₃ or AlBr₃ to form a halogenoarene, exemplified by benzene to form chlorobenzene and methylbenzene to form 2-chloromethylbenzene and 4-chloromethylbenzene
- explain the difference in reactivity between a halogenoalkane and a halogenoarene as exemplified by chloroethane and chlorobenzene
Preparation of Halogenoarenes
Halogenoarenes are prepared by electrophilic substitution reactions of arenes with chlorine or bromine. You must be able to recall the reactions, reagents and conditions by which halogenoarenes are produced.
General Reaction Conditions
Halogenation of the aromatic ring occurs under conditions that favour electrophilic substitution.
- \( \mathrm{Cl_2} \) or \( \mathrm{Br_2} \)
- halogen carrier catalyst: AlCl₃ or AlBr₃
- room temperature
- absence of UV light
The catalyst generates a strong electrophile, allowing substitution of a hydrogen atom on the benzene ring while preserving the delocalised π system.
Chlorination of Benzene
Benzene reacts with chlorine in the presence of aluminium chloride to form chlorobenzene.
\( \mathrm{C_6H_6 + Cl_2 \xrightarrow{AlCl_3} C_6H_5Cl + HCl} \)
This is an electrophilic substitution reaction in which a chlorine atom replaces a hydrogen atom on the ring.
Chlorination of Methylbenzene
Methylbenzene reacts with chlorine under the same conditions, but the methyl group activates the ring and directs substitution to the ortho and para positions.
As a result, a mixture of isomers is formed.
Major products:
\( \mathrm{C_6H_5CH_3 + Cl_2 \xrightarrow{AlCl_3}} \)
- 2-chloromethylbenzene (ortho-chlorotoluene)
- 4-chloromethylbenzene (para-chlorotoluene)
The para isomer usually predominates due to reduced steric hindrance.
Example
State the reagents and conditions needed to convert benzene into chlorobenzene.
▶️ Answer / Explanation
Chlorine in the presence of aluminium chloride at room temperature.
Example
When methylbenzene reacts with chlorine in the presence of AlCl₃, two major products are formed. Name these products and explain why both are produced.
▶️ Answer / Explanation
The products are 2-chloromethylbenzene and 4-chloromethylbenzene.
The methyl group is an electron-donating substituent that directs electrophilic substitution to the ortho and para positions on the benzene ring.
Reactivity of Halogenoalkanes and Halogenoarenes
Halogenoalkanes and halogenoarenes both contain a carbon–halogen bond, but they show very different reactivity.
Observed Difference in Reactivity
Chloroethane reacts readily in nucleophilic substitution reactions.
Chlorobenzene is largely unreactive under the same conditions.
Bonding in Chloroethane
In chloroethane, the carbon bonded to chlorine is sp³ hybridised.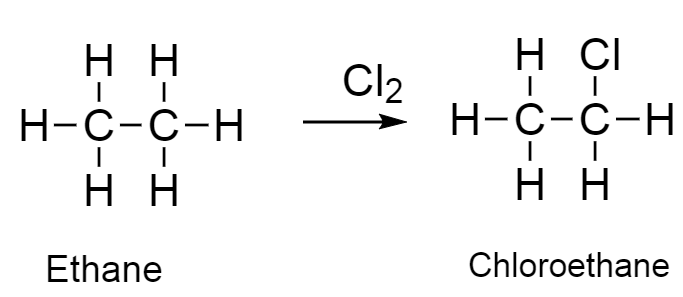
The C–Cl bond is a normal σ bond that is relatively weak and polar.
As a result:
- the carbon atom is partially positive
- the C–Cl bond can be broken heterolytically
- chloride ions can act as leaving groups
This allows chloroethane to undergo nucleophilic substitution reactions such as hydrolysis.
Bonding in Chlorobenzene
In chlorobenzene, the carbon bonded to chlorine is part of a benzene ring and is sp² hybridised.
The lone pair on the chlorine atom overlaps with the delocalised π system of the benzene ring.
This causes the C–Cl bond to have partial double bond character.
Consequences of Delocalisation
- the C–Cl bond in chlorobenzene is shorter and stronger
- the bond is more difficult to break
- chloride is a poor leaving group
In addition, the delocalised π system makes the benzene ring resistant to nucleophilic attack.
Overall Comparison
Chloroethane reacts because it has a polar C–Cl σ bond that can be broken easily.
Chlorobenzene does not react under similar conditions because the C–Cl bond is strengthened by delocalisation into the aromatic ring.
Example
Chloroethane reacts readily with aqueous hydroxide ions, but chlorobenzene does not. State one reason for this difference.
▶️ Answer / Explanation
In chlorobenzene, the C–Cl bond has partial double bond character due to delocalisation.
This makes the bond stronger and harder to break than the C–Cl bond in chloroethane.
Example
Explain, with reference to bonding and structure, why chlorobenzene is resistant to nucleophilic substitution whereas chloroethane is not.
▶️ Answer / Explanation
In chloroethane, the carbon atom is sp³ hybridised and the C–Cl bond is a polar σ bond, which can be broken heterolytically.
In chlorobenzene, the carbon atom is sp² hybridised and the lone pair on chlorine overlaps with the delocalised π system.
This gives the C–Cl bond partial double bond character, making it shorter and stronger, so nucleophilic substitution does not occur readily.
