CIE AS/A Level Chemistry 32.1 Alcohols Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 32.1 Alcohols Study Notes – New Syllabus
CIE AS/A Level Chemistry 32.1 Alcohols Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- describe the reaction with acyl chlorides to form esters using ethyl ethanoate
Reaction of Acyl Chlorides to Form Esters
Acyl chlorides react readily with alcohols to form esters. You must be able to describe this reaction, including reagents, conditions and products, as exemplified by the formation of ethyl ethanoate.
General Reaction
An acyl chloride reacts with an alcohol to form an ester and hydrogen chloride.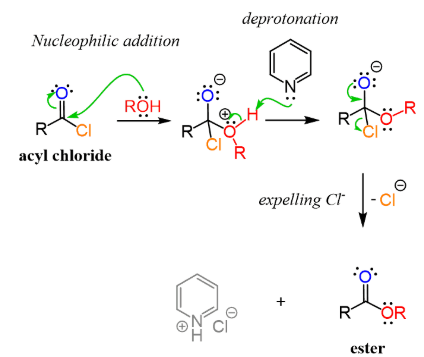
\( \mathrm{RCOCl + R’OH \rightarrow RCOOR’ + HCl} \)
The reaction is rapid, occurs at room temperature and does not require a catalyst.
Example: Formation of Ethyl Ethanoate
Ethyl ethanoate is formed when ethanoyl chloride reacts with ethanol.
\( \mathrm{CH_3COCl + CH_3CH_2OH \rightarrow CH_3COOCH_2CH_3 + HCl} \)
The ester formed is ethyl ethanoate, and hydrogen chloride gas is released.
Reaction Mechanism
This reaction proceeds via an addition–elimination mechanism.
- the alcohol acts as a nucleophile and attacks the electron-deficient carbonyl carbon
- a tetrahedral intermediate is formed
- chloride ions are eliminated as a leaving group
Acyl chlorides are very reactive due to the polar C–Cl bond and the strongly electron-withdrawing chlorine atom.
Key Features of the Reaction
- fast reaction at room temperature
- produces a steamy white mist of HCl fumes
- irreversible under normal conditions
Example
Name the ester formed when ethanoyl chloride reacts with ethanol.
▶️ Answer / Explanation
The acyl chloride provides the ethanoate part of the name.
The alcohol provides the ethyl part of the name.
The ester formed is ethyl ethanoate.
Example
Describe and explain the reaction between ethanoyl chloride and ethanol.
▶️ Answer / Explanation
Ethanoyl chloride reacts rapidly with ethanol at room temperature to form ethyl ethanoate and hydrogen chloride.
The reaction occurs by an addition–elimination mechanism.
Ethanol acts as a nucleophile and attacks the carbonyl carbon, and chloride ions are eliminated as a leaving group.
