CIE AS/A Level Chemistry 33.1 Carboxylic acids Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 33.1 Carboxylic acids Study Notes – New Syllabus
CIE AS/A Level Chemistry 33.1 Carboxylic acids Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reaction by which benzoic acid can be produced:
(a) reaction of an alkylbenzene with hot alkaline KMnO₄ and then dilute acid, exemplified by
methylbenzenedescribe the reaction of carboxylic acids with PCl₃ and heat, PCl₅ or SOCl₂ to form acyl chlorides
recognise that some carboxylic acids can be further oxidised:
(a) the oxidation of methanoic acid, HCOOH, with Fehling’s reagent or Tollens’ reagent or acidified
KMnO₄ or acidified K₂Cr₂O₇ to carbon dioxide and water
(b) the oxidation of ethanedioic acid, HOOCCOOH, with warm acidified KMnO₄ to carbon dioxidedescribe and explain the relative acidities of carboxylic acids, phenols and alcohols
describe and explain the relative acidities of chlorine-substituted carboxylic acids
Preparation of Benzoic Acid from Alkylbenzenes
Benzoic acid can be produced by the oxidation of an alkylbenzene. You must be able to recall the reagents and conditions for this reaction, as exemplified by the conversion of methylbenzene into benzoic acid.
Reaction Overview
An alkyl side-chain attached to a benzene ring can be completely oxidised to a carboxylic acid group.

The benzene ring itself remains unchanged.
Reagents and Conditions
- hot alkaline \( \mathrm{KMnO_4} \) (reflux)
- then acidify with dilute acid (e.g. dilute \( \mathrm{HCl} \))
Example: Methylbenzene → Benzoic Acid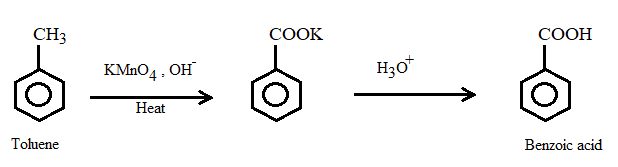
Methylbenzene is oxidised by hot alkaline potassium manganate(VII).
\( \mathrm{C_6H_5CH_3 \xrightarrow[\text{reflux}]{KMnO_4/\,OH^-} C_6H_5COO^-} \)
On acidification, the benzoate ion is converted into benzoic acid.
\( \mathrm{C_6H_5COO^- + H^+ \rightarrow C_6H_5COOH} \)
Key Observations
- purple \( \mathrm{KMnO_4} \) is decolourised
- a brown precipitate of \( \mathrm{MnO_2} \) may form
- a white solid of benzoic acid forms on acidification
Important Exam Points
Any alkylbenzene (with at least one hydrogen on the side-chain carbon) is oxidised to benzoic acid, regardless of the length of the side-chain.
The oxidation affects only the side-chain; the aromatic ring is resistant due to its delocalised π system.
Example
State the reagents and conditions needed to convert methylbenzene into benzoic acid.
▶️ Answer / Explanation
Heat under reflux with alkaline potassium manganate(VII), then acidify with a dilute acid.
Example
Explain why oxidation of methylbenzene with hot alkaline \( \mathrm{KMnO_4} \) produces benzoic acid rather than breaking the benzene ring.
▶️ Answer / Explanation
The benzene ring is stabilised by a delocalised π electron system and is resistant to oxidation.
The alkyl side-chain is more reactive and is completely oxidised to a carboxyl group.
Conversion of Carboxylic Acids to Acyl Chlorides
Carboxylic acids can be converted into acyl chlorides by reaction with PCl₃, PCl₅ or SOCl₂. You must be able to describe the reagents, conditions and products of these reactions.
General Reaction
In each case, the –OH group of the carboxylic acid is replaced by a –Cl group, forming an acyl chloride.
\( \mathrm{RCOOH \rightarrow RCOCl} \)
Reaction with PCl₃
Carboxylic acids react with phosphorus trichloride on heating to form acyl chlorides.
Equation:
\( \mathrm{3RCOOH + PCl_3 \rightarrow 3RCOCl + H_3PO_3} \)
This reaction requires heat and produces phosphorous acid as a by-product.
Reaction with PCl₅
Phosphorus pentachloride reacts vigorously with carboxylic acids at room temperature.
Equation:
\( \mathrm{RCOOH + PCl_5 \rightarrow RCOCl + POCl_3 + HCl} \)
Steamy white fumes of hydrogen chloride are produced.
Reaction with SOCl₂
Thionyl chloride is commonly used because its by-products are gases.
Equation:
\( \mathrm{RCOOH + SOCl_2 \rightarrow RCOCl + SO_2 + HCl} \)
Both by-products, \( \mathrm{SO_2} \) and \( \mathrm{HCl} \), are gases and escape, driving the reaction to completion.
Comparison of Reagents
- PCl₃: requires heat, produces a liquid by-product
- PCl₅: reacts vigorously, produces HCl fumes
- SOCl₂: preferred reagent due to gaseous by-products
Key Exam Points
These reactions are used because acyl chlorides are more reactive than carboxylic acids and are useful intermediates in synthesis.
Example
State a reagent that can be used to convert a carboxylic acid into an acyl chloride.
▶️ Answer / Explanation
Thionyl chloride, \( \mathrm{SOCl_2} \).
Example
Explain why thionyl chloride is often preferred to phosphorus pentachloride for the preparation of acyl chlorides.
▶️ Answer / Explanation
Thionyl chloride produces sulfur dioxide and hydrogen chloride as gaseous by-products.
These gases escape, driving the reaction to completion and making purification easier.
Further Oxidation of Certain Carboxylic Acids
Although most carboxylic acids are resistant to oxidation, some carboxylic acids can be further oxidised. You must be able to recognise which acids undergo oxidation and recall the reagents, conditions and products.
Key Idea
Carboxylic acids that already contain a carbonyl carbon at its highest oxidation state are normally resistant to oxidation.
However, acids such as methanoic acid and ethanedioic acid are exceptions and can be oxidised further to carbon dioxide.
(a) Oxidation of Methanoic Acid, \( \mathrm{HCOOH} \)
Methanoic acid behaves as a reducing agent and can be oxidised to carbon dioxide and water. 
It can be oxidised by several common oxidising agents:
- Fehling’s solution (warm)
- Tollens’ reagent (warm)
- acidified \( \mathrm{KMnO_4} \)
- acidified \( \mathrm{K_2Cr_2O_7} \)
Overall oxidation:
\( \mathrm{HCOOH \rightarrow CO_2 + H_2O} \)
This is unusual because other carboxylic acids do not reduce Fehling’s or Tollens’ reagents.
(b) Oxidation of Ethanedioic Acid, \( \mathrm{HOOCCOOH} \)
Ethanedioic acid (oxalic acid) can be oxidised by warm acidified potassium manganate(VII).
Conditions:
- warm
- acidified \( \mathrm{KMnO_4} \)
Reaction:
\( \mathrm{HOOCCOOH \rightarrow 2CO_2 + 2H^+ + 2e^-} \)
The purple colour of \( \mathrm{KMnO_4} \) is decolourised as it is reduced.
Why These Acids Can Be Oxidised
Methanoic acid contains a hydrogen atom bonded directly to the carboxyl carbon, allowing further oxidation.
Ethanedioic acid readily decomposes into carbon dioxide due to the presence of two adjacent carboxyl groups.
Key Exam Statements
- most carboxylic acids are resistant to oxidation
- methanoic acid is an exception and acts as a reducing agent
- ethanedioic acid is oxidised by warm acidified \( \mathrm{KMnO_4} \)
Example
State the products formed when methanoic acid is oxidised by Tollens’ reagent.
▶️ Answer / Explanation
Carbon dioxide and water.
Example
Explain why methanoic acid, but not ethanoic acid, gives a positive result with Fehling’s solution.
▶️ Answer / Explanation
Methanoic acid can be oxidised further because it contains a hydrogen atom attached to the carboxyl carbon.
It acts as a reducing agent and reduces Fehling’s solution.
Ethanoic acid cannot be further oxidised and therefore does not react.
Relative Acidities of Carboxylic Acids, Phenols and Alcohols
Carboxylic acids, phenols and alcohols can all act as acids, but they differ significantly in acid strength. You must be able to describe and explain their relative acidities by comparing the stability of their conjugate bases.
Order of Acidity
The relative acidities are:
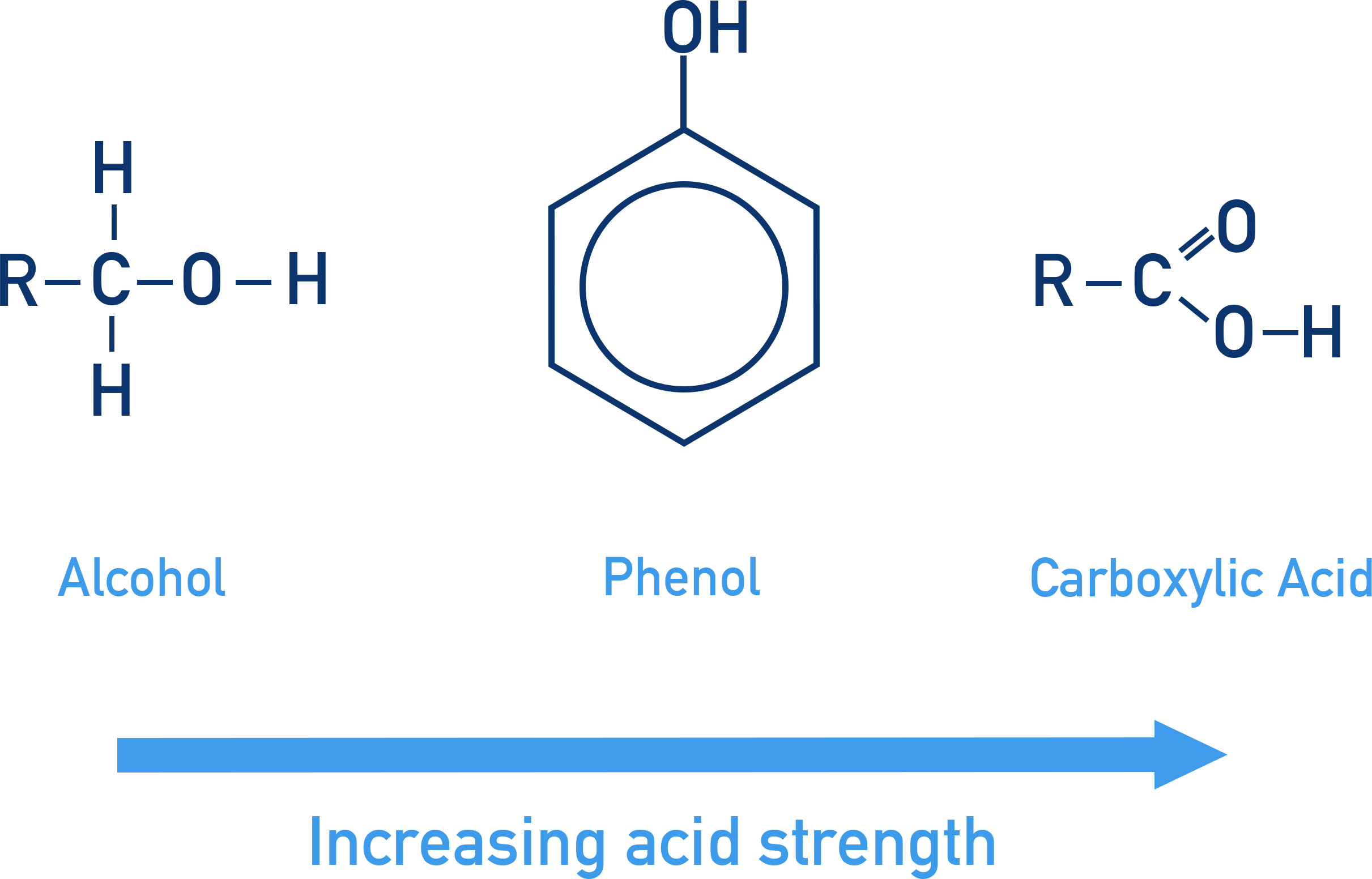
General Principle
Acid strength depends on the stability of the conjugate base formed when a proton is lost.
The more stable the conjugate base, the stronger the acid.
Carboxylic Acids
Carboxylic acids lose a proton to form a carboxylate ion:
\( \mathrm{RCOOH \rightleftharpoons RCOO^- + H^+} \)
The carboxylate ion is highly stabilised because:
- the negative charge is delocalised over two oxygen atoms
- both oxygen atoms are highly electronegative
- the charge is spread evenly by resonance
This strong stabilisation makes carboxylic acids the most acidic of the three.
Phenols
Phenols lose a proton to form a phenoxide ion:
\( \mathrm{ArOH \rightleftharpoons ArO^- + H^+} \)
The phenoxide ion is stabilised by:
- delocalisation of the negative charge into the aromatic ring
- overlap with the delocalised π system
However, the negative charge is largely on one oxygen atom and only partially delocalised, so phenols are less acidic than carboxylic acids.
Alcohols
Alcohols lose a proton to form an alkoxide ion:
\( \mathrm{ROH \rightleftharpoons RO^- + H^+} \)
The alkoxide ion is least stable because:
- the negative charge is localised on one oxygen atom
- alkyl groups have a +I (electron-donating) effect
- electron donation destabilises the negative charge
Therefore, alcohols are the least acidic of the three.
Summary Explanation
Carboxylic acids are most acidic due to strong delocalisation over two oxygen atoms.
Phenols are moderately acidic due to delocalisation into the aromatic ring.
Alcohols are least acidic because their conjugate bases are destabilised by alkyl groups.
Example
Arrange ethanol, phenol and ethanoic acid in order of increasing acidity and give one reason.
▶️ Answer / Explanation
Ethanol < phenol < ethanoic acid.
Ethanoic acid is most acidic because its conjugate base is stabilised by delocalisation over two oxygen atoms.
Example
Explain why phenol is more acidic than an alcohol but less acidic than a carboxylic acid.
▶️ Answer / Explanation
Phenol forms a phenoxide ion that is stabilised by delocalisation into the aromatic ring, making it more acidic than an alcohol.
However, a carboxylate ion is more stable because the negative charge is delocalised over two oxygen atoms, making carboxylic acids stronger acids than phenols.
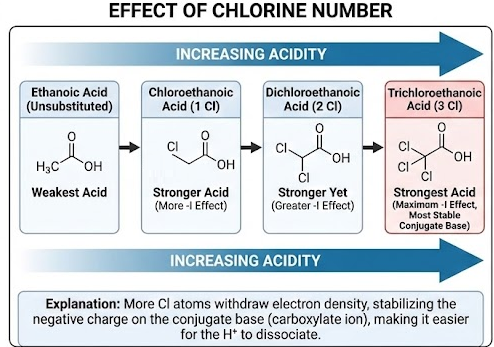
Relative Acidities of Chlorine-Substituted Carboxylic Acids
Chlorine-substituted carboxylic acids are stronger acids than unsubstituted carboxylic acids. You must be able to describe and explain how the number and position of chlorine atoms affect acidity.
General Order of Acidity
As the number of chlorine atoms increases, acidity increases:
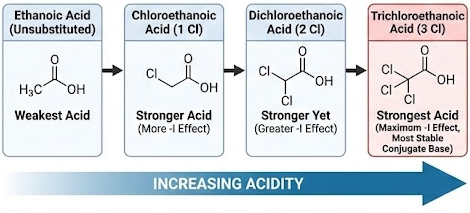
Reason: Stability of the Conjugate Base
Carboxylic acids lose a proton to form a carboxylate ion:
\( \mathrm{RCOOH \rightleftharpoons RCOO^- + H^+} \)
The strength of the acid depends on how stable the carboxylate ion is.
Inductive (–I) Effect of Chlorine
Chlorine is highly electronegative and has a strong –I (electron-withdrawing) effect.
This effect:
- pulls electron density away from the carboxylate ion
- reduces the concentration of negative charge on the oxygen atoms

- stabilises the conjugate base
Greater stabilisation of the conjugate base means a stronger acid.
Effect of Number of Chlorine Atoms
Each additional chlorine atom increases the overall electron-withdrawing effect.
Therefore:
- chloroethanoic acid is stronger than ethanoic acid
- dichloroethanoic acid is stronger than chloroethanoic acid
- trichloroethanoic acid is the strongest
Effect of Position of Chlorine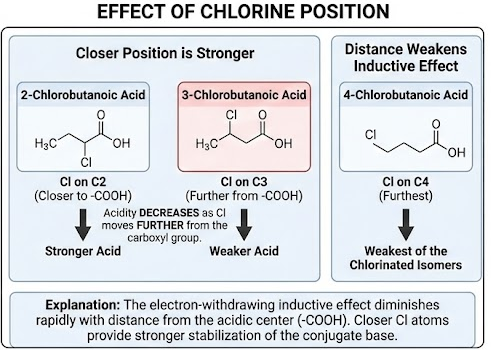
The inductive effect decreases with distance from the carboxyl group.
Chlorine atoms closer to the –COOH group have a greater acid-strengthening effect.
For example:
2-chloropropanoic acid > 3-chloropropanoic acid
Key Exam Statement
Chlorine-substituted carboxylic acids are more acidic because chlorine withdraws electron density by an inductive effect, stabilising the carboxylate ion.
Example
Explain why chloroethanoic acid is more acidic than ethanoic acid.
▶️ Answer / Explanation
Chlorine has a –I electron-withdrawing effect.
It pulls electron density away from the carboxylate ion, stabilising the negative charge.
This makes chloroethanoic acid more acidic than ethanoic acid.
Example
Explain the order of acidity: ethanoic acid < chloroethanoic acid < dichloroethanoic acid.
▶️ Answer / Explanation
Each chlorine atom has an electron-withdrawing inductive effect.
Increasing the number of chlorine atoms increases stabilisation of the carboxylate ion.
Greater stabilisation of the conjugate base leads to greater acidity.