CIE AS/A Level Chemistry 33.3 Acyl chlorides Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 33.3 Acyl chlorides Study Notes – New Syllabus
CIE AS/A Level Chemistry 33.3 Acyl chlorides Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which acyl chlorides can be produced:
(a) reaction of carboxylic acids with PCl₃ and heat, PCl₅ or SOCl₂describe the following reactions of acyl chlorides:
(a) hydrolysis on addition of water at room temperature to give the carboxylic acid and HCl
(b) reaction with an alcohol at room temperature to produce an ester and HCl
(c) reaction with phenol at room temperature to produce an ester and HCl
(d) reaction with ammonia at room temperature to produce an amide and HCl
(e) reaction with a primary or secondary amine at room temperature to produce an amide and HCldescribe the addition–elimination mechanism of acyl chlorides in reactions in 33.3.2(a)–(e)
explain the relative ease of hydrolysis of acyl chlorides, alkyl chlorides and halogenoarenes
(aryl chlorides)
Preparation of Acyl Chlorides
Acyl chlorides can be produced from carboxylic acids by replacing the –OH group with a –Cl group.
General Change
\( \mathrm{RCOOH \rightarrow RCOCl} \)
This reaction is carried out because acyl chlorides are much more reactive than carboxylic acids and are useful intermediates in synthesis.
(a) Reaction with Phosphorus Trichloride, \( \mathrm{PCl_3} \)
Carboxylic acids react with phosphorus trichloride on heating.
Equation:
\( \mathrm{3RCOOH + PCl_3 \rightarrow 3RCOCl + H_3PO_3} \)
This reaction requires heat and produces phosphorous acid as a by-product.
(b) Reaction with Phosphorus Pentachloride, \( \mathrm{PCl_5} \)
Phosphorus pentachloride reacts vigorously with carboxylic acids at room temperature.
Equation:
\( \mathrm{RCOOH + PCl_5 \rightarrow RCOCl + POCl_3 + HCl} \)
Steamy white fumes of hydrogen chloride are observed.
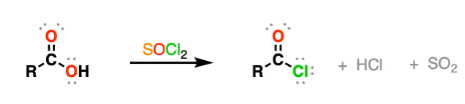
(c) Reaction with Thionyl Chloride, \( \mathrm{SOCl_2} \)
Thionyl chloride is commonly used because its by-products are gases.
Equation:
\( \mathrm{RCOOH + SOCl_2 \rightarrow RCOCl + SO_2 + HCl} \)
The gaseous by-products, \( \mathrm{SO_2} \) and \( \mathrm{HCl} \), escape, helping to drive the reaction to completion and simplifying purification.
Comparison of Reagents (Recall)
- PCl₃ → heat required, liquid by-product formed
- PCl₅ → reacts at room temperature, HCl fumes produced
- SOCl₂ → preferred reagent, gaseous by-products
Key Exam Statement
Carboxylic acids are converted into acyl chlorides using PCl₃ and heat, PCl₅, or SOCl₂.
Example
State one reagent that can be used to convert a carboxylic acid into an acyl chloride.
▶️ Answer / Explanation
Thionyl chloride, \( \mathrm{SOCl_2} \).
Example
Describe how ethanoic acid can be converted into ethanoyl chloride, including reagents and conditions.
▶️ Answer / Explanation
Ethanoic acid can be reacted with phosphorus trichloride on heating, or with phosphorus pentachloride at room temperature, or with thionyl chloride.
In each case, the –OH group is replaced by –Cl to form ethanoyl chloride.
Reactions of Acyl Chlorides
Acyl chlorides are highly reactive derivatives of carboxylic acids. Their reactivity arises from the polar C–Cl bond and the electron-deficient carbonyl carbon. You must be able to describe the reactions of acyl chlorides with water, alcohols, phenols and amines, including reagents, conditions and products.
General Reaction Type
All reactions of acyl chlorides proceed by an addition–elimination mechanism.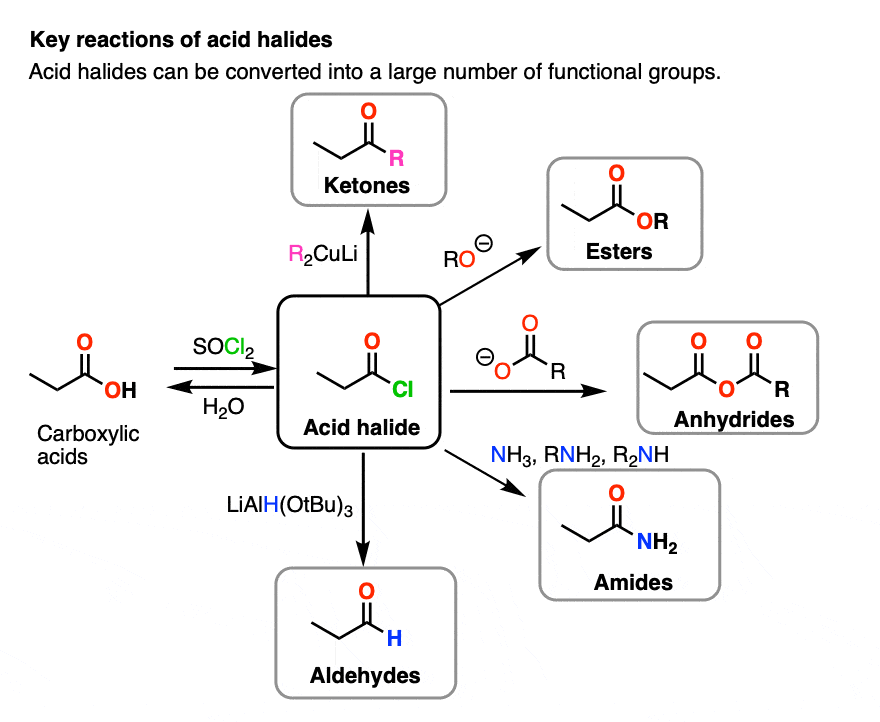
A nucleophile attacks the carbonyl carbon, followed by elimination of a chloride ion.
(a) Hydrolysis with Water
Acyl chlorides react violently with water at room temperature.
\( \mathrm{RCOCl + H_2O \rightarrow RCOOH + HCl} \)
The products are a carboxylic acid and hydrogen chloride. Steamy white fumes of HCl are observed.
(b) Reaction with an Alcohol
Acyl chlorides react readily with alcohols at room temperature.
\( \mathrm{RCOCl + R’OH \rightarrow RCOOR’ + HCl} \)
The product is an ester. This reaction is rapid and does not require a catalyst.
(c) Reaction with Phenol
Acyl chlorides also react with phenols at room temperature.
\( \mathrm{RCOCl + ArOH \rightarrow RCOOAr + HCl} \)
The product is an aryl ester, and hydrogen chloride is released.
(d) Reaction with Ammonia
Acyl chlorides react with ammonia at room temperature to form an amide.
\( \mathrm{RCOCl + NH_3 \rightarrow RCONH_2 + HCl} \)
In practice, excess ammonia is used so that the HCl produced is neutralised to form ammonium chloride.
(e) Reaction with Primary or Secondary Amines
Acyl chlorides react readily with primary or secondary amines at room temperature.
General equations:
Primary amine:
\( \mathrm{RCOCl + R’NH_2 \rightarrow RCONHR’ + HCl} \)
Secondary amine:
\( \mathrm{RCOCl + R’_2NH \rightarrow RCONR’_2 + HCl} \)
The products are N-substituted amides. Excess amine is usually added to neutralise the HCl formed.
All reactions of acyl chlorides occur at room temperature and produce hydrogen chloride as a by-product.
Example
Describe what happens when ethanoyl chloride is added to water.
▶️ Answer / Explanation
Ethanoyl chloride reacts rapidly with water to form ethanoic acid and hydrogen chloride.
Steamy white fumes of HCl are observed.
Example
Explain why excess ammonia is used when reacting an acyl chloride with ammonia.
▶️ Answer / Explanation
The reaction produces hydrogen chloride as a by-product.
Excess ammonia neutralises the HCl to form ammonium chloride, preventing the amide product from being protonated.
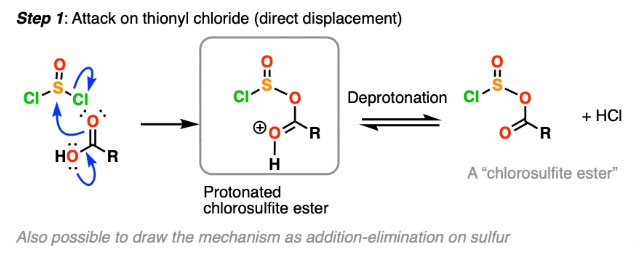
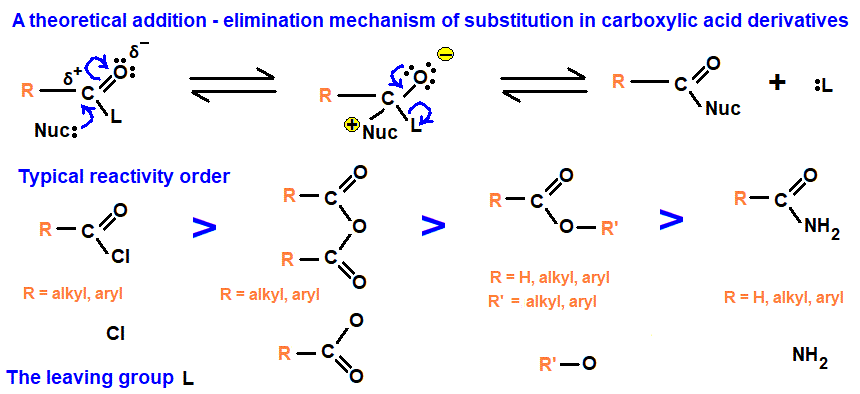
Addition–Elimination Mechanism of Acyl Chlorides
All reactions of acyl chlorides with water, alcohols, phenols, ammonia and amines proceed via the same fundamental mechanism: the addition–elimination mechanism.
Why Acyl Chlorides Are So Reactive
Acyl chlorides contain a highly polar C=O bond and a polar C–Cl bond.
This makes the carbonyl carbon electron deficient (δ⁺) and very susceptible to attack by nucleophiles.
General Addition–Elimination Mechanism
The mechanism occurs in two main steps:
- nucleophilic addition to the carbonyl carbon
- elimination of chloride ion
Step 1: Nucleophilic Addition
A nucleophile (e.g. \( \mathrm{H_2O} \), \( \mathrm{ROH} \), \( \mathrm{ArOH} \), \( \mathrm{NH_3} \), or an amine) attacks the carbonyl carbon.
The π bond of the C=O breaks, and a tetrahedral intermediate is formed.
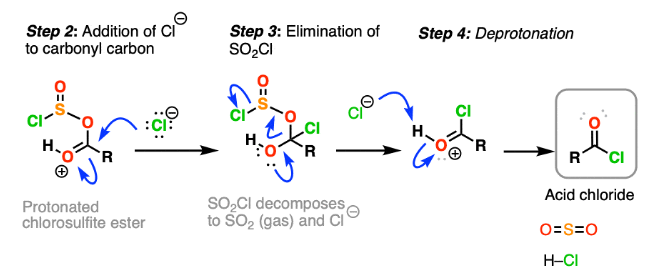
Step 2: Elimination
The tetrahedral intermediate is unstable.
The C=O bond reforms and a chloride ion is eliminated, restoring the carbonyl group.
This produces an acyl substitution product.
Final Proton Transfer
If the nucleophile is neutral (e.g. water or alcohol), a proton is lost to form HCl.
Application to Reactions (a)–(e)
(a) Hydrolysis with Water
Nucleophile: \( \mathrm{H_2O} \)
Product: carboxylic acid + HCl
Water adds to the carbonyl carbon, followed by elimination of Cl⁻ and proton loss.
(b) Reaction with an Alcohol
Nucleophile: \( \mathrm{ROH} \)
Product: ester + HCl
An alkoxide group becomes attached to the acyl carbon.
(c) Reaction with Phenol
Nucleophile: phenol (or phenoxide if base is present)
Product: aryl ester + HCl
The mechanism is identical to alcohols but proceeds slightly more slowly.
(d) Reaction with Ammonia
Nucleophile: \( \mathrm{NH_3} \)
Product: amide + HCl
Excess ammonia is used to neutralise the HCl formed.
(e) Reaction with Primary or Secondary Amines
Nucleophile: primary or secondary amine
Product: N-substituted amide + HCl
Amines are stronger nucleophiles than alcohols, so these reactions are very fast.
Acyl chlorides undergo nucleophilic addition followed by elimination of Cl⁻. This addition–elimination mechanism applies to reactions with water, alcohols, phenols and amines.
Example
Identify the nucleophile in the reaction between an acyl chloride and ethanol.
▶️ Answer / Explanation
Ethanol acts as the nucleophile and attacks the carbonyl carbon.
Example
Describe the addition–elimination mechanism for the reaction of an acyl chloride with ammonia.
▶️ Answer / Explanation
Ammonia acts as a nucleophile and attacks the electron-deficient carbonyl carbon.
A tetrahedral intermediate is formed.
The carbonyl bond reforms and chloride ions are eliminated.
A proton is lost, forming an amide and hydrogen chloride.
Relative Ease of Hydrolysis of Acyl Chlorides, Alkyl Chlorides and Aryl Chlorides
Halogen-containing organic compounds show very different reactivities towards hydrolysis with water. You must be able to explain the relative ease of hydrolysis of acyl chlorides, alkyl chlorides and halogenoarenes (aryl chlorides) in terms of bond polarity, structure and reaction mechanism.
Overall Order of Reactivity
acyl chlorides >> alkyl chlorides >>> aryl chlorides
(a) Acyl Chlorides – Very Rapid Hydrolysis
Acyl chlorides hydrolyse violently with water at room temperature:
\( \mathrm{RCOCl + H_2O \rightarrow RCOOH + HCl} \)
This rapid hydrolysis is due to:
- a highly polar C–Cl bond
- a strongly electron-deficient carbonyl carbon
- the carbonyl group which activates the molecule towards nucleophilic attack
- chloride ions being an excellent leaving group
Hydrolysis occurs by an addition–elimination mechanism, which has a low activation energy.
(b) Alkyl Chlorides – Slow Hydrolysis
Alkyl chlorides hydrolyse much more slowly and usually require heating with aqueous hydroxide.
Reasons for slower hydrolysis:
- the C–Cl bond is only moderately polar
- the carbon atom is sp³ hybridised
- hydrolysis occurs by nucleophilic substitution (SN1 or SN2)
Water is a weak nucleophile, so hydrolysis is slow compared with acyl chlorides.
(c) Aryl Chlorides – Very Resistant to Hydrolysis
Halogenoarenes such as chlorobenzene are extremely resistant to hydrolysis.
This is because:
- the carbon–chlorine bond has partial double-bond character
- lone pairs on chlorine are delocalised into the aromatic π system
- the C–Cl bond is shorter and stronger
- the carbon atom is sp² hybridised
As a result:
- the C–Cl bond is difficult to break
- chloride is a poor leaving group
- nucleophilic substitution is unfavourable
Comparison Summary
- Acyl chlorides hydrolyse readily due to a highly activated carbonyl carbon and a good leaving group.
- Alkyl chlorides hydrolyse slowly because water is a weak nucleophile and the C–Cl bond is less polar.
- Aryl chlorides are resistant to hydrolysis due to partial double-bond character and delocalisation.
Example
State which compound hydrolyses most readily: an acyl chloride or an alkyl chloride.
▶️ Answer / Explanation
An acyl chloride hydrolyses most readily because it contains an activated carbonyl carbon and reacts by an addition–elimination mechanism.
Example
Explain why chlorobenzene does not hydrolyse under the same conditions as chloroethane.
▶️ Answer / Explanation
In chlorobenzene, the chlorine lone pair is delocalised into the aromatic ring, giving the C–Cl bond partial double-bond character.
This makes the bond stronger and prevents nucleophilic substitution, so hydrolysis does not occur.
