CIE AS/A Level Chemistry 34.1 Primary and secondary amines Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 34.1 Primary and secondary amines Study Notes – New Syllabus
CIE AS/A Level Chemistry 34.1 Primary and secondary amines Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which primary and secondary amines are produced:
(a) reaction of halogenoalkanes with NH₃ in ethanol heated under pressure
(b) reaction of halogenoalkanes with primary amines in ethanol, heated in a sealed tube/under
pressure
(c) the reduction of amides with LiAlH₄
(d) the reduction of nitriles with LiAlH₄ or H₂/Nidescribe the condensation reaction of ammonia or an amine with an acyl chloride at room
temperature to give an amidedescribe and explain the basicity of aqueous solutions of amines
Preparation of Primary and Secondary Amines
Primary and secondary amines can be prepared by a range of synthetic routes. You must be able to recall the reagents and conditions for each method and identify the type of amine produced.
(a) Halogenoalkanes + Ammonia
Halogenoalkanes react with excess ammonia to form a primary amine.
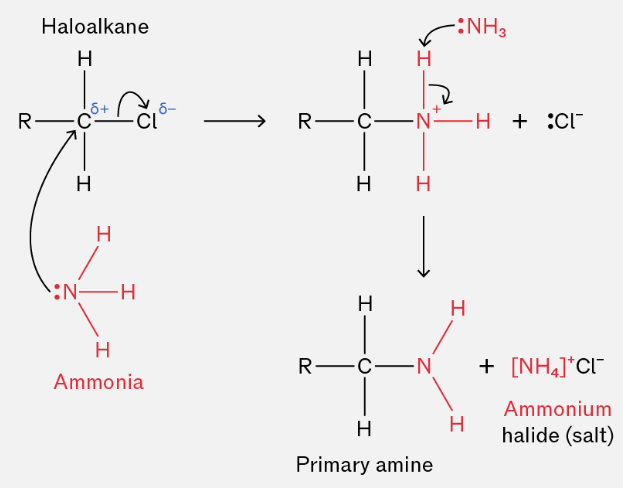
Reagents and conditions:
- halogenoalkane
- excess NH₃ in ethanol
- heat under pressure / sealed tube
\( \mathrm{R{-}X + NH_3 \rightarrow R{-}NH_2 + HX} \)
Excess ammonia is used to reduce further substitution and favour formation of the primary amine.
(b) Halogenoalkanes + Primary Amines
Halogenoalkanes react with primary amines to form secondary amines.
Reagents and conditions:
- halogenoalkane
- primary amine in ethanol
- heat under pressure / sealed tube
\( \mathrm{R{-}X + R'{-}NH_2 \rightarrow R'{-}NHR + HX} \)
This reaction also proceeds by nucleophilic substitution.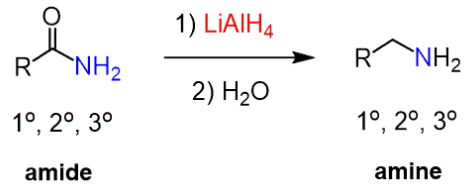
(c) Reduction of Amides
Amides can be reduced to form amines.
Reagents and conditions:
- LiAlH₄ in dry ether
- followed by aqueous work-up
\( \mathrm{RCONH_2 \rightarrow RCH_2NH_2} \)
A primary amide produces a primary amine.
(d) Reduction of Nitriles
Nitriles can be reduced to form primary amines.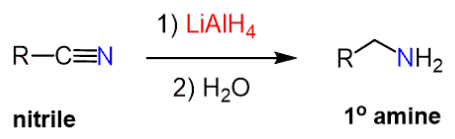
Two possible methods:
- LiAlH₄ in dry ether, then aqueous work-up
- H₂ / Ni catalyst and heat
\( \mathrm{RCN \rightarrow RCH_2NH_2} \)
This method increases the carbon chain length by one carbon.
Key Points
- halogenoalkane + NH₃ → primary amine
- halogenoalkane + primary amine → secondary amine
- amides reduced by LiAlH₄ → amines
- nitriles reduced → primary amines
Example
State the reagents and conditions needed to prepare ethylamine from bromoethane.
▶️ Answer / Explanation
Bromoethane is heated with excess ammonia in ethanol under pressure.
Example
Describe how a secondary amine could be prepared from a halogenoalkane.
▶️ Answer / Explanation
The halogenoalkane is heated with a primary amine in ethanol in a sealed tube or under pressure.
The reaction occurs by nucleophilic substitution to form a secondary amine.
Formation of Amides from Acyl Chlorides
Acyl chlorides react readily with ammonia or amines at room temperature to form amides. You must be able to describe this condensation reaction, including reagents, conditions and products.
General Reaction
An acyl chloride reacts with ammonia or an amine to form an amide with the elimination of hydrogen chloride.
\( \mathrm{RCOCl + NH_3 \rightarrow RCONH_2 + HCl} \)
\( \mathrm{RCOCl + R’NH_2 \rightarrow RCONHR’ + HCl} \)
This is a condensation reaction because a small molecule (HCl) is eliminated.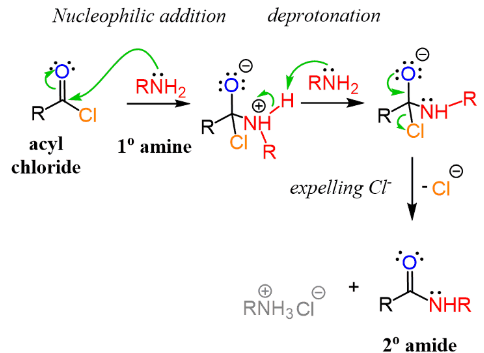
Reagents and Conditions
- acyl chloride
- ammonia or amine
- room temperature
- often excess ammonia or amine is used
No catalyst or heating is required because acyl chlorides are highly reactive.
Products Formed
The type of amide formed depends on the nitrogen-containing reagent:
- ammonia → primary amide
- primary amine → secondary amide
- secondary amine → tertiary amide
The hydrogen chloride produced is often neutralised by excess ammonia or amine, forming an ammonium salt.
Reaction Mechanism
The reaction proceeds via an addition–elimination mechanism.
- the amine or ammonia acts as a nucleophile
- it attacks the electron-deficient carbonyl carbon
- a tetrahedral intermediate forms
- chloride ions are eliminated as a leaving group
Example
Name the product formed when ethanoyl chloride reacts with ammonia.
▶️ Answer / Explanation
Ethanoyl chloride reacts with ammonia to form ethanamide.
Example
Describe and explain the reaction between propanoyl chloride and methylamine.
▶️ Answer / Explanation
Propanoyl chloride reacts with methylamine at room temperature to form a secondary amide and hydrogen chloride.
The reaction occurs by an addition–elimination mechanism in which methylamine acts as a nucleophile and chloride ions are eliminated as a leaving group.
Basicity of Aqueous Solutions of Amines
Amines are bases and form alkaline aqueous solutions. You must be able to describe and explain the basicity of amines in water in terms of the lone pair of electrons on the nitrogen atom.
Why Amines Are Basic
Amines contain a nitrogen atom with a lone pair of electrons.
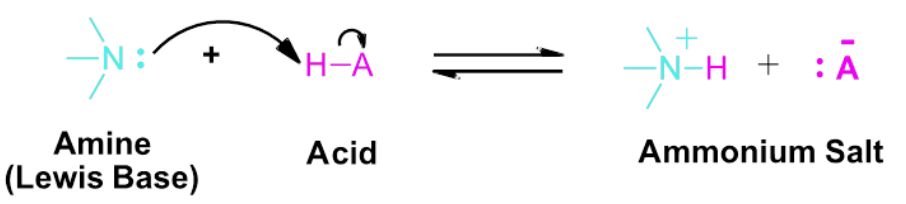
This lone pair can be used to accept a proton, so amines act as Brønsted–Lowry bases.
Reaction with Water
In aqueous solution, an amine reacts with water:
\( \mathrm{RNH_2 + H_2O \rightleftharpoons RNH_3^+ + OH^-} \)
The formation of hydroxide ions explains why aqueous amine solutions are alkaline.
Strength of the Base
Amines are weak bases.
The equilibrium lies to the left, meaning that only a fraction of amine molecules accept a proton at any one time.
Effect of Alkyl Groups
Alkyl groups have a +I (electron-donating) effect.
This pushes electron density towards the nitrogen atom, making the lone pair more available to accept a proton.
As a result:
- amines are more basic than ammonia
- alkyl-substituted amines generally form more alkaline solutions than ammonia
Formation of Ammonium Salts
When an amine reacts with an acid, an ammonium salt is formed.
\( \mathrm{RNH_2 + HCl \rightarrow RNH_3^+Cl^-} \)
This further demonstrates the basic nature of amines.
Aqueous solutions of amines are basic because the lone pair on nitrogen accepts protons from water, producing hydroxide ions.
Example
Explain why aqueous ethylamine has a pH greater than 7.
▶️ Answer / Explanation
Ethylamine contains a nitrogen atom with a lone pair of electrons.
The lone pair accepts a proton from water, forming ethylammonium ions and hydroxide ions.
The presence of hydroxide ions makes the solution alkaline.
Example
Compare the basicity of ammonia and methylamine in aqueous solution.
▶️ Answer / Explanation
Both ammonia and methylamine act as bases by accepting a proton via the lone pair on nitrogen.
Methylamine is more basic because the methyl group has a +I effect, increasing electron density on the nitrogen atom.
This makes the lone pair more available to accept a proton.
