CIE AS/A Level Chemistry 34.3 Amides Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 34.3 Amides Study Notes – New Syllabus
CIE AS/A Level Chemistry 34.3 Amides Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recall the reactions (reagents and conditions) by which amides are produced:
(a) the reaction between ammonia and an acyl chloride at room temperature
(b) the reaction between a primary amine and an acyl chloride at room temperaturedescribe the reactions of amides:
(a) hydrolysis with aqueous alkali or aqueous acid
(b) the reduction of the CO group in amides with LiAlH₄ to form an aminestate and explain why amides are much weaker bases than amines
Preparation of Amides
Amides can be prepared by reactions of acyl chlorides with ammonia or primary amines. You must be able to recall the reagents and conditions for these reactions and identify the type of amide formed.
(a) Ammonia + Acyl Chloride
An acyl chloride reacts readily with ammonia at room temperature to form a primary amide.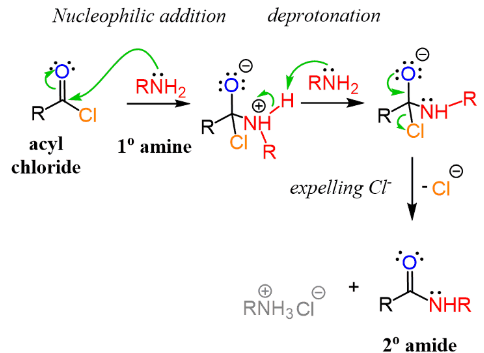
Reagents and conditions:
- acyl chloride
- ammonia (often in excess)
- room temperature
\( \mathrm{RCOCl + NH_3 \rightarrow RCONH_2 + HCl} \)
The hydrogen chloride produced is neutralised by excess ammonia, forming ammonium chloride.
(b) Primary Amine + Acyl Chloride
An acyl chloride also reacts with a primary amine at room temperature to form a secondary amide.
Reagents and conditions:
- acyl chloride
- primary amine (often in excess)
- room temperature
\( \mathrm{RCOCl + R’NH_2 \rightarrow RCONHR’ + HCl} \)
The hydrogen chloride formed is neutralised by excess amine, producing an alkylammonium chloride.
Nature of the Reactions
Both reactions are condensation reactions and proceed by an addition–elimination mechanism.
No heating or catalyst is required because acyl chlorides are highly reactive.
Key Points
- acyl chloride + ammonia → primary amide
- acyl chloride + primary amine → secondary amide
- both reactions occur at room temperature
- HCl is produced and neutralised by excess base
Example
State the reagents needed to prepare ethanamide from ethanoyl chloride.
▶️ Answer / Explanation
Ethanoyl chloride reacted with ammonia at room temperature.
Example
Describe how a secondary amide can be prepared from an acyl chloride.
▶️ Answer / Explanation
The acyl chloride is reacted with a primary amine at room temperature.
The reaction forms a secondary amide and hydrogen chloride, which is neutralised by excess amine.
Reactions of Amides
Amides are relatively unreactive compounds due to resonance stabilisation of the –CONH– group. You must be able to describe the reactions of amides, including hydrolysis and reduction, stating reagents, conditions and products.
(a) Hydrolysis of Amides
Amides can be hydrolysed by heating with either aqueous alkali or aqueous acid.
Hydrolysis with Aqueous Alkali
When heated under reflux with aqueous sodium hydroxide, an amide is hydrolysed to form a carboxylate ion and ammonia.
\( \mathrm{RCONH_2 + OH^- \rightarrow RCOO^- + NH_3} \)
This reaction is irreversible because ammonia escapes as a gas.
Hydrolysis with Aqueous Acid
When heated under reflux with a dilute acid (e.g. dilute HCl), an amide is hydrolysed to form a carboxylic acid and an ammonium salt.
\( \mathrm{RCONH_2 + H_2O + H^+ \rightarrow RCOOH + NH_4^+} \)
The ammonium ion remains in solution as an ammonium salt.
Why Harsh Conditions Are Needed
Amides are resistant to hydrolysis because the lone pair on nitrogen is delocalised into the carbonyl group.
This gives the C–N bond partial double bond character, making it more difficult to break.
(b) Reduction of Amides
Amides can be reduced to amines using lithium aluminium hydride.
Reagents and conditions:
- LiAlH₄ in dry ether
- followed by aqueous work-up
\( \mathrm{RCONH_2 \rightarrow RCH_2NH_2} \)
The carbonyl (C=O) group is reduced to a –CH₂– group, forming a primary amine.
Key Points
- amides require heating under reflux for hydrolysis
- alkaline hydrolysis gives a carboxylate ion and ammonia
- acidic hydrolysis gives a carboxylic acid and ammonium ions
- LiAlH₄ reduces amides to amines
Example
Describe the products formed when ethanamide is heated under reflux with aqueous sodium hydroxide.
▶️ Answer / Explanation
Ethanamide is hydrolysed to give ethanoate ions and ammonia.
The reaction is irreversible because ammonia is released.
Example
Describe how propanamide can be converted into propylamine, stating reagents and conditions.
▶️ Answer / Explanation
Propanamide is reduced using lithium aluminium hydride in dry ether, followed by aqueous work-up.
The carbonyl group is reduced to a methylene group, producing propylamine.
Basicity of Amides Compared with Amines
Amides are much weaker bases than amines. You must be able to state and explain this difference in basicity with reference to the availability of the nitrogen lone pair.
Statement
Amides are much weaker bases than amines because the lone pair on the nitrogen atom is less available to accept a proton.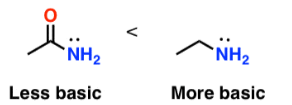
Explanation
In amines, the lone pair on nitrogen is localised and readily available to accept a proton.
As a result, amines react with water and acids to form ammonium ions.
Delocalisation in Amides
In amides, the nitrogen atom is bonded to a carbonyl group (C=O).
The lone pair on nitrogen is delocalised into the carbonyl group, forming a resonance structure with a C=N bond.
This delocalisation gives the C–N bond partial double bond character.
Effect on Basicity
- the nitrogen lone pair is less available to accept a proton
- protonation of nitrogen would disrupt the stabilising delocalisation
- amides therefore behave as very weak bases
Example
State one reason why an amide is less basic than an amine.
▶️ Answer / Explanation
In an amide, the lone pair on nitrogen is delocalised into the carbonyl group, making it less available to accept a proton.
Example
Explain why ethanamide does not behave as a base in aqueous solution in the same way as ethylamine.
▶️ Answer / Explanation
In ethanamide, the nitrogen lone pair is delocalised into the carbonyl group, giving the C–N bond partial double bond character.
This delocalisation stabilises the amide and makes the lone pair unavailable for protonation.
In ethylamine, the lone pair is localised and readily accepts a proton, so it behaves as a base.
