CIE AS/A Level Chemistry 34.4 Amino acids Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 34.4 Amino acids Study Notes – New Syllabus
CIE AS/A Level Chemistry 34.4 Amino acids Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe the acid/base properties of amino acids and the formation of zwitterions, to include the isoelectric point
describe the formation of amide (peptide) bonds between amino acids to give di- and tripeptides
interpret and predict the results of electrophoresis on mixtures of amino acids and dipeptides at varying pHs (the assembling of the apparatus will not be tested)
Acid–Base Properties of Amino Acids and Zwitterions
Amino acids show both acidic and basic behaviour because they contain two functional groups. You must be able to describe the acid–base properties of amino acids, explain the formation of zwitterions, and define the isoelectric point.
Functional Groups in Amino Acids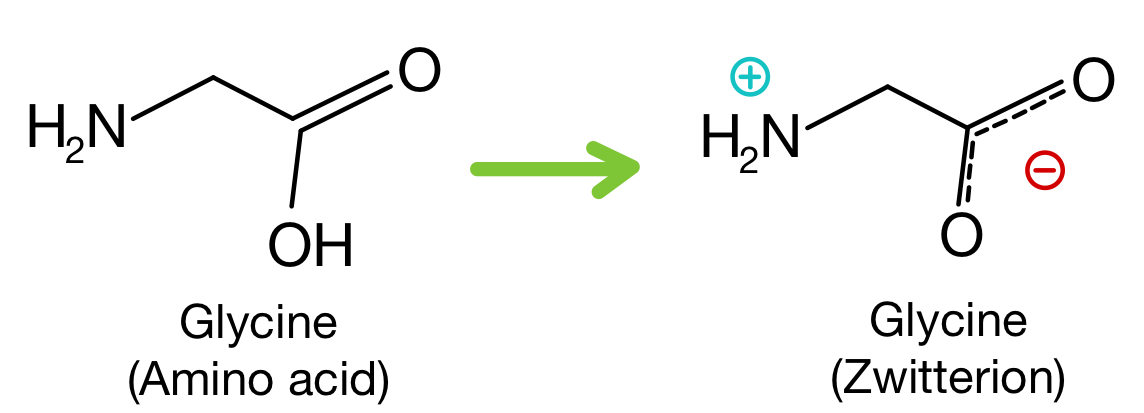
All amino acids (except proline) contain:
- an amino group, –NH₂ (basic)
- a carboxyl group, –COOH (acidic)
Because they can act as both acids and bases, amino acids are described as amphoteric.
Acid–Base Behaviour
In acidic solution:
The amino group accepts a proton and becomes –NH₃⁺.
In alkaline solution:
The carboxyl group loses a proton and becomes –COO⁻.
Formation of Zwitterions
In aqueous solution, amino acids mainly exist as zwitterions.
A zwitterion contains both a positive and a negative charge within the same molecule:
–NH₃⁺ and –COO⁻
Zwitterions have:
- no overall charge
- strong ionic interactions
- high melting points and good water solubility
Reaction with Acids
When an amino acid is added to acid:
the –COO⁻ group accepts a proton to form –COOH.
The molecule has an overall positive charge.
Reaction with Bases
When an amino acid is added to alkali:
the –NH₃⁺ group loses a proton to form –NH₂.
The molecule has an overall negative charge.
Isoelectric Point
The isoelectric point, \( \mathrm{pI} \), is the pH at which an amino acid:
- exists predominantly as a zwitterion
- has no net charge
- does not migrate in an electric field
At the isoelectric point, amino acids have their lowest solubility in water.
Summary of pH Effects
Low pH → overall positive ion
Isoelectric point → zwitterion (no net charge)
High pH → overall negative ion
Example
Explain why amino acids are described as amphoteric.
▶️ Answer / Explanation
Amino acids contain an acidic carboxyl group and a basic amino group.
As a result, they can both donate and accept protons.
Example
Explain the meaning of the isoelectric point of an amino acid.
▶️ Answer / Explanation
The isoelectric point is the pH at which the amino acid exists mainly as a zwitterion.
At this pH, the molecule has no net charge and does not move in an electric field.
Formation of Amide (Peptide) Bonds in Amino Acids
Amino acids join together to form peptides and proteins by forming amide bonds, more commonly known in biological systems as peptide bonds. You must be able to describe how dipeptides and tripeptides are formed.
The Peptide (Amide) Bond
A peptide bond is an amide linkage with the functional group: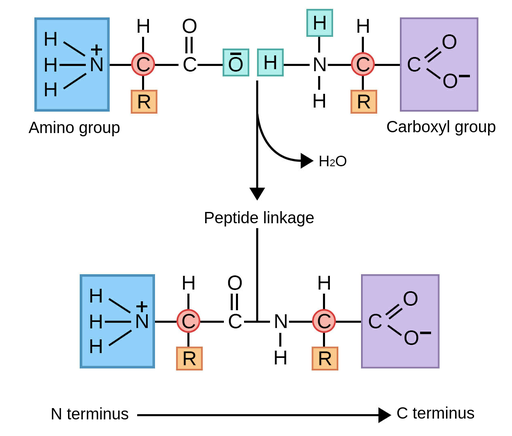
–CONH–
It forms when the carboxyl group of one amino acid reacts with the amino group of another.
Condensation Reaction
Peptide bond formation is a condensation reaction.
During the reaction:
- the –OH from the carboxyl group is removed
- a hydrogen atom is removed from the –NH₂ group
- one molecule of water is eliminated
Formation of a Dipeptide
When two amino acids react together, a dipeptide is formed.
One peptide bond is formed and one molecule of water is released.
General representation:
\( \mathrm{H_2N{-}CHR{-}COOH + H_2N{-}CHR'{-}COOH \rightarrow H_2N{-}CHR{-}CONH{-}CHR'{-}COOH + H_2O} \)
Formation of a Tripeptide
When three amino acids join together, a tripeptide is formed.
This involves the formation of two peptide bonds and the elimination of two molecules of water.
Direction of Peptide Chains
Peptides have a direction:
- N-terminus → free –NH₂ group
- C-terminus → free –COOH group
This is important when naming and drawing peptides.
Key Points
- peptide bonds are amide bonds
- formed by condensation between –COOH and –NH₂ groups
- dipeptide → two amino acids, one peptide bond
- tripeptide → three amino acids, two peptide bonds
- each peptide bond formation releases one molecule of water
Example
Explain how a dipeptide is formed from two amino acids.
▶️ Answer / Explanation
A dipeptide is formed when the carboxyl group of one amino acid reacts with the amino group of another.
A peptide (amide) bond is formed and one molecule of water is eliminated in a condensation reaction.
Example
Describe the formation of a tripeptide from three amino acids.
▶️ Answer / Explanation
Three amino acids join together by two condensation reactions.
Each reaction forms a peptide bond between a carboxyl group and an amino group.
Two molecules of water are eliminated, producing a tripeptide with two peptide bonds.
Electrophoresis of Amino Acids and Dipeptides
Electrophoresis is a technique used to separate amino acids and dipeptides based on their net charge. You must be able to interpret and predict the results of electrophoresis at different pH values.
Principle of Electrophoresis
In an electric field:

- positively charged species move towards the cathode (–)
- negatively charged species move towards the anode (+)
- species with no net charge do not move
The charge on an amino acid or dipeptide depends on the pH of the solution.
Effect of pH on Charge
Low pH (Acidic Solution)
In acidic conditions:
- –NH₂ groups are protonated to –NH₃⁺
- –COOH groups remain as –COOH
- overall charge is positive
Amino acids and dipeptides therefore move towards the cathode.
High pH (Alkaline Solution)
In alkaline conditions:
- –COOH groups lose protons to form –COO⁻
- –NH₃⁺ groups lose protons to form –NH₂
- overall charge is negative
Amino acids and dipeptides therefore move towards the anode.
Isoelectric Point (pI)![]()
At the isoelectric point:
- the molecule exists mainly as a zwitterion
- it has no net charge
- it does not move during electrophoresis
Comparing Amino Acids and Dipeptides
Dipeptides contain:
- one free –NH₂ / –NH₃⁺ group (N-terminus)
- one free –COOH / –COO⁻ group (C-terminus)
They therefore behave similarly to amino acids, but may have a different isoelectric point depending on their side chains.
Predicting Electrophoresis Results
To predict movement:
- identify the pH of the buffer
- compare the pH with the pI of each amino acid or dipeptide
- determine the net charge
- predict the direction of movement
Summary
pH < pI → moves to cathode
pH = pI → no movement
pH > pI → moves to anode
Example
An amino acid has a pI of 6.0. Predict its movement in an electrophoresis experiment at pH 3.
▶️ Answer / Explanation
At pH 3 (below the pI), the amino acid has an overall positive charge.
It will move towards the cathode.
Example
A mixture contains an amino acid (pI = 5.5) and a dipeptide (pI = 8.0). Describe and explain what happens when electrophoresis is carried out at pH 7.
▶️ Answer / Explanation
At pH 7, the amino acid (pI 5.5) has a net negative charge and moves towards the anode.
The dipeptide (pI 8.0) has a net positive charge and moves towards the cathode.
The two substances separate because they have different net charges at this pH.
