CIE AS/A Level Chemistry 35.1 Condensation polymerisation Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 35.1 Condensation polymerisation Study Notes – New Syllabus
CIE AS/A Level Chemistry 35.1 Condensation polymerisation Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe the formation of polyesters:
(a) the reaction between a diol and a dicarboxylic acid or dioyl chloride
(b) the reaction of a hydroxycarboxylic aciddescribe the formation of polyamides:
(a) the reaction between a diamine and a dicarboxylic acid or dioyl chloride
(b) the reaction of an aminocarboxylic acid
(c) the reaction between amino acidsdeduce the repeat unit of a condensation polymer obtained from a given monomer or pair of
monomersidentify the monomer(s) present in a given section of a condensation polymer molecule
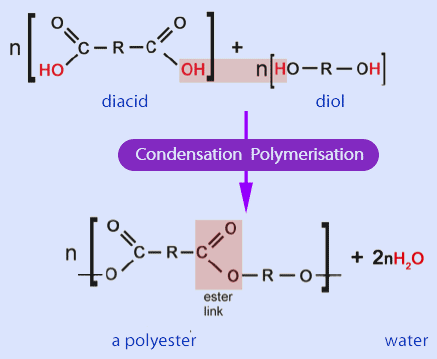
Formation of Polyesters
Polyesters are formed by condensation polymerisation reactions that produce ester linkages (–COO–). You must be able to describe how polyesters are formed by two different routes and identify the reagents, conditions and repeating units.
The Ester Linkage
All polyesters contain the ester functional group:
–COO–
Each ester linkage forms with the elimination of a small molecule.
(a) Diol + Dicarboxylic Acid or Dioyl Chloride

(i) Diol + Dicarboxylic Acid
A diol reacts with a dicarboxylic acid to form a polyester.
Reagents and conditions:
- diol (two –OH groups)
- dicarboxylic acid (two –COOH groups)
- heat
- acid catalyst often used
Each ester bond forms with the elimination of water.
General representation:
\( \mathrm{nHO{-}R{-}OH + nHOOC{-}R'{-}COOH \rightarrow [{-}O{-}R{-}OCO{-}R'{-}CO{-}]_n + 2nH_2O} \)
(ii) Diol + Dioyl Chloride
A diol reacts with a dioyl chloride much more readily.

Reagents and conditions:
- diol
- dioyl chloride
- room temperature
Each ester linkage forms with the elimination of HCl.
This reaction occurs without heating because acyl chlorides are very reactive.
(b) Hydroxycarboxylic Acid
A hydroxycarboxylic acid contains both:
- a hydroxyl group, –OH
- a carboxyl group, –COOH
These molecules can undergo self-condensation polymerisation.
Reagents and conditions:
- hydroxycarboxylic acid
- heat
Each ester linkage forms with the elimination of water.
General representation:
\( \mathrm{nHO{-}R{-}COOH \rightarrow [{-}O{-}R{-}CO{-}]_n + nH_2O} \)
Example
State the type of polymerisation and the small molecule eliminated when a diol reacts with a dicarboxylic acid.
▶️ Answer / Explanation
Condensation polymerisation occurs and water is eliminated.
Example
Explain why polyesters can be formed from hydroxycarboxylic acids without adding a second monomer.
▶️ Answer / Explanation
A hydroxycarboxylic acid contains both an –OH group and a –COOH group.
These functional groups can react with each other to form ester linkages.
Repeated condensation reactions produce a polyester and eliminate water.
Formation of Polyamides
Polyamides are polymers that contain repeating amide (–CONH–) linkages. They are formed by condensation polymerisation reactions. You must be able to describe how polyamides are formed by different routes and identify the reagents, conditions and small molecules eliminated.
The Amide (Peptide) Linkage
All polyamides contain the functional group:
–CONH–
Each amide linkage forms with the elimination of a small molecule.
(a) Diamine + Dicarboxylic Acid or Dioyl Chloride
(i) Diamine + Dicarboxylic Acid
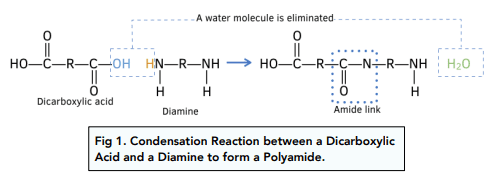
A diamine reacts with a dicarboxylic acid to form a polyamide.
Reagents and conditions:
- diamine (two –NH₂ groups)
- dicarboxylic acid (two –COOH groups)
- strong heating
Each amide bond forms with the elimination of water.
General representation:
\( \mathrm{nH_2N{-}R{-}NH_2 + nHOOC{-}R'{-}COOH \rightarrow [{-}NH{-}R{-}NHCO{-}R'{-}CO{-}]_n + 2nH_2O} \)
(ii) Diamine + Dioyl Chloride
A diamine reacts very readily with a dioyl chloride.
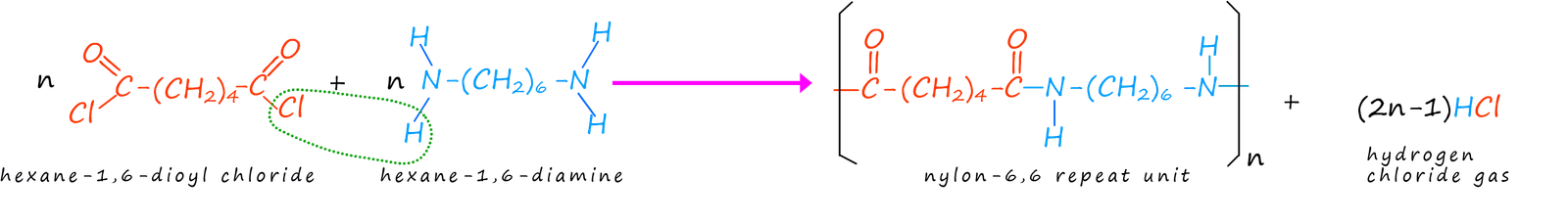
Reagents and conditions:
- diamine
- dioyl chloride
- room temperature
Each amide linkage forms with the elimination of HCl.
This reaction occurs easily because acyl chlorides are highly reactive.
(b) Aminocarboxylic Acid
An aminocarboxylic acid contains both:
- an amino group, –NH₂
- a carboxyl group, –COOH
These molecules undergo self-condensation polymerisation.
Reagents and conditions:
- aminocarboxylic acid
- heat
Each amide bond forms with the elimination of water.
General representation:
\( \mathrm{nH_2N{-}R{-}COOH \rightarrow [{-}NH{-}R{-}CO{-}]_n + nH_2O} \)
(c) Reaction Between Amino Acids
Amino acids are aminocarboxylic acids.
They can react together to form polyamides, known biologically as polypeptides or proteins.
The amide links formed are called peptide bonds.
Each peptide bond forms by a condensation reaction with the loss of water.
Key Points
- polyamides form by condensation polymerisation
- all polyamides contain the –CONH– linkage
- diamine + dicarboxylic acid → water eliminated
- diamine + dioyl chloride → HCl eliminated
- aminocarboxylic acids and amino acids self-polymerise
Example
State the small molecule eliminated when a diamine reacts with a dicarboxylic acid to form a polyamide.
▶️ Answer / Explanation
Water is eliminated.
Example
Explain why amino acids can form polyamides without the need for a second monomer.
▶️ Answer / Explanation
Amino acids contain both an amino group and a carboxyl group.
These functional groups can react together to form amide (peptide) bonds.
Repeated condensation reactions produce a polyamide with the elimination of water.
Deducing the Repeat Unit of a Condensation Polymer
In condensation polymerisation, monomers join together with the elimination of a small molecule such as water or hydrogen chloride. You must be able to deduce the repeat unit of a condensation polymer from:
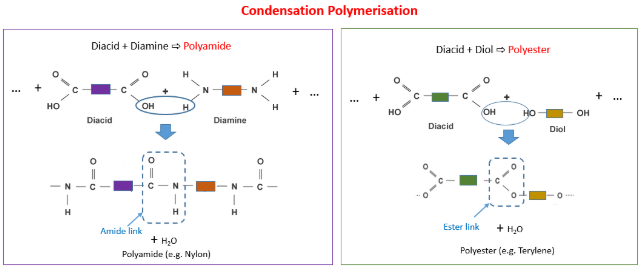
- a single monomer
- a pair of monomers
Key Skill
To deduce a repeat unit, you must:
- identify the functional groups involved
- identify the link formed (–COO– or –CONH–)
- remove the atoms lost during condensation
- draw the smallest section that repeats
1. Polyesters (–COO– link)
(a) From Two Monomers: Diol + Dicarboxylic Acid
Monomers:
diol: \( \mathrm{HO{-}R{-}OH} \)
dicarboxylic acid: \( \mathrm{HOOC{-}R'{-}COOH} \)
Each ester bond forms with the loss of H₂O.
Repeat unit:
–O–R–OCO–R′–CO–
(b) From One Monomer: Hydroxycarboxylic Acid
Monomer:
\( \mathrm{HO{-}R{-}COOH} \)
The –OH and –COOH groups react within different molecules.
Repeat unit:
–O–R–CO–
2. Polyamides (–CONH– link)
(a) From Two Monomers: Diamine + Dicarboxylic Acid
Monomers:
diamine: \( \mathrm{H_2N{-}R{-}NH_2} \)
dicarboxylic acid: \( \mathrm{HOOC{-}R'{-}COOH} \)
Each amide bond forms with the loss of H₂O.
Repeat unit:
–NH–R–NHCO–R′–CO–
(b) From One Monomer: Aminocarboxylic Acid
Monomer:
\( \mathrm{H_2N{-}R{-}COOH} \)
Each amide bond forms with the loss of H₂O.
Repeat unit:
–NH–R–CO–
Technique:
Step 1: Circle the functional groups that react
Step 2: Draw the bond that forms between monomers
Step 3: Remove H₂O or HCl
Step 4: Put brackets around the repeating section
Errors to Avoid
- forgetting to remove the small molecule
- including –OH or –NH₂ end groups in the repeat unit
- drawing the repeat unit too large
Example
A polymer is formed from hexane-1,6-diol and hexanedioic acid. Deduce the repeat unit of the polymer.
▶️ Answer / Explanation
The polymer is a polyester formed by condensation.
The repeat unit is:
–O–(CH₂)₆–OCO–(CH₂)₄–CO–
Example
A polymer is formed from 6-aminohexanoic acid. Deduce the repeat unit.
▶️ Answer / Explanation
6-aminohexanoic acid contains an –NH₂ group and a –COOH group.
It forms a polyamide by self-condensation.
The repeat unit is:
–NH–(CH₂)₅–CO–
Identifying the Monomer(s) from a Condensation Polymer Segment
You must be able to identify the monomer or monomers used to form a condensation polymer when you are given a section of its polymer chain. This requires recognising the linkage present and reconstructing the original functional groups.
Key Idea
Condensation polymers are formed when monomers join with the elimination of a small molecule (usually \( \mathrm{H_2O} \) or \( \mathrm{HCl} \)).

To identify the monomer(s), you must reverse the condensation.
Step-by-Step Method
- Identify the functional group linkage in the polymer
- Split the polymer chain at this linkage
- Add back the atoms lost during condensation
- Identify whether there was one monomer or two different monomers
1. Polyesters (–COO– linkage)
If the polymer contains an ester linkage, –COO–:
Cut the chain at –COO– and add:
- –OH to the oxygen side
- –COOH to the carbonyl side
Two-Monomer Polyester
If alternating units are present, the monomers are:
- a diol
- a dicarboxylic acid (or dioyl chloride)
One-Monomer Polyester
If the same unit repeats on both sides, the monomer is a:
hydroxycarboxylic acid
2. Polyamides (–CONH– linkage)
If the polymer contains an amide (peptide) linkage, –CONH–:
Cut the chain at –CONH– and add:
- –NH₂ to the nitrogen side
- –COOH to the carbonyl side
Two-Monomer Polyamide
- a diamine
- a dicarboxylic acid (or dioyl chloride)
One-Monomer Polyamide
If the same group can supply both –NH₂ and –COOH, the monomer is an:
aminocarboxylic acid (including amino acids)
Fast Rule
–COO– present → polyester → diol + diacid OR hydroxycarboxylic acid
–CONH– present → polyamide → diamine + diacid OR aminocarboxylic acid
Example
A polymer segment contains the linkage –O–(CH₂)₄–OCO–(CH₂)₂–CO–. Identify the monomers used.
▶️ Answer / Explanation
The –COO– linkage shows the polymer is a polyester.
The monomers are butane-1,4-diol and ethanedioic acid.
Example
A polymer contains the repeating unit –NH–(CH₂)₅–CO–. Identify the monomer.
▶️ Answer / Explanation
The –CONH– linkage shows the polymer is a polyamide.
Only one carbon chain is present, so it must come from a single monomer.
The monomer is 6-aminohexanoic acid.
