CIE AS/A Level Chemistry 35.2 Predicting the type of polymerisation Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 35.2 Predicting the type of polymerisation Study Notes – New Syllabus
CIE AS/A Level Chemistry 35.2 Predicting the type of polymerisation Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
predict the type of polymerisation reaction for a given monomer or pair of monomers
deduce the type of polymerisation reaction which produces a given section of a polymer molecule
Predicting the Type of Polymerisation
We must be able to predict the type of polymerisation reaction that will occur when you are given a monomer or a pair of monomers. This depends on the functional groups present in the molecule(s).
The Two Types of Polymerisation
- Addition polymerisation
- Condensation polymerisation
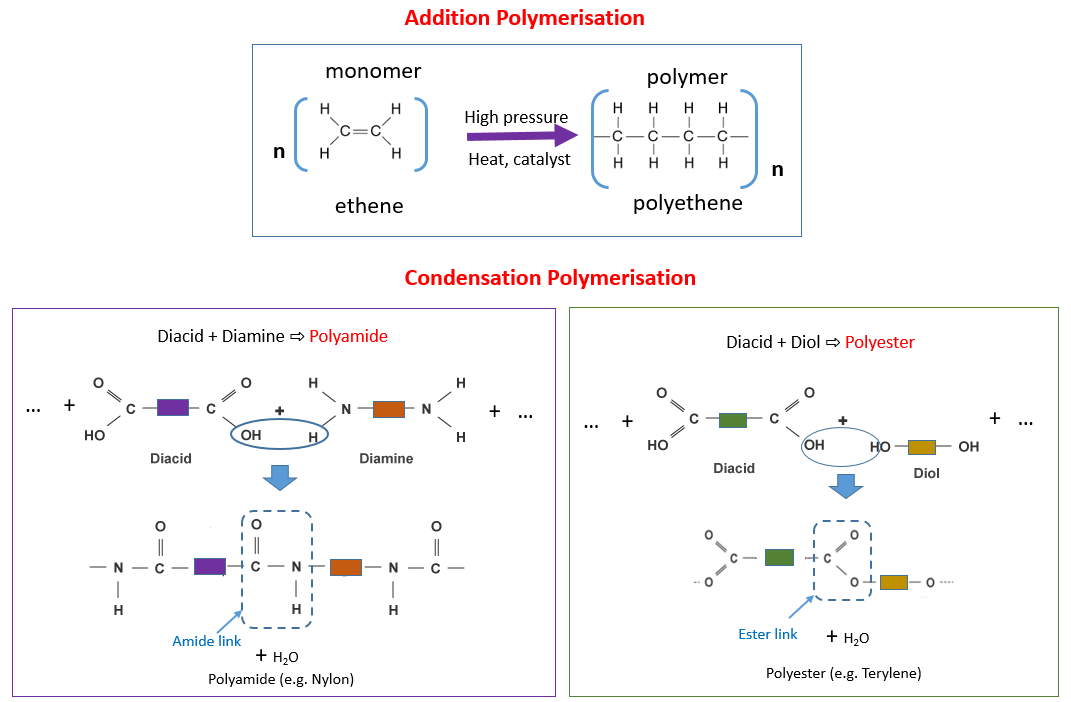
1. Addition Polymerisation
Addition polymerisation occurs when monomers contain a carbon–carbon double bond, usually in an alkene.
Key features:
- monomer contains C=C
- one type of monomer
- no small molecule is eliminated
- product is an addition polymer
Example monomer:
\( \mathrm{CH_2{=}CH_2} \)
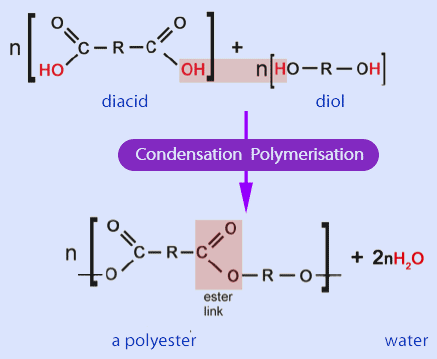
2. Condensation Polymerisation
Condensation polymerisation occurs when monomers contain two functional groups that can react together.
Key features:
- monomers contain two functional groups
- often two different monomers, or one bifunctional monomer
- a small molecule is eliminated (usually \( \mathrm{H_2O} \) or \( \mathrm{HCl} \))
- product is a condensation polymer
How to Predict the Type
Step 1: Look for a C=C bond
If present → addition polymerisation
Step 2: If no C=C bond, look for two functional groups
–OH, –COOH, –NH₂
If present → condensation polymerisation
Typical Condensation Polymer Pairs
- diol + dicarboxylic acid → polyester
- diol + dioyl chloride → polyester
- diamine + dicarboxylic acid → polyamide
- diamine + dioyl chloride → polyamide
- hydroxycarboxylic acid → polyester
- aminocarboxylic acid / amino acids → polyamide
Summary
C=C present → addition polymerisation
Two functional groups present → condensation polymerisation
Example
Predict the type of polymerisation for hex-1-ene.
▶️ Answer / Explanation
Hex-1-ene contains a carbon–carbon double bond.
It undergoes addition polymerisation.
Example
A polymer is formed from a diamine and a dicarboxylic acid. Predict the type of polymerisation and name the type of polymer formed.
▶️ Answer / Explanation
The monomers each contain two functional groups.
Condensation polymerisation occurs with elimination of water.
The polymer formed is a polyamide.
Deducing the Type of Polymerisation from a Polymer Section
The structure of the polymer tells you how it was formed. In particular, look for functional groups that indicate whether a small molecule was eliminated during polymerisation.
1. Addition Polymerisation
A polymer has been formed by addition polymerisation if:
- the polymer backbone contains only C–C single bonds
- there are no heteroatoms (O or N) in the main chain
- the repeat unit can be traced back to an alkene monomer
This type of polymerisation involves no elimination of small molecules.
Typical backbone:
–CH₂–CH(R)–
2. Condensation Polymerisation
A polymer has been formed by condensation polymerisation if:
- the chain contains heteroatoms such as O or N
- there are ester (–COO–) or amide (–CONH–) linkages
- the polymer must have been formed with elimination of a small molecule (usually \( \mathrm{H_2O} \) or \( \mathrm{HCl} \))

Polyester Evidence
Presence of –COO– in the backbone → condensation polymerisation
Polyamide Evidence
Presence of –CONH– in the backbone → condensation polymerisation
Fast Exam Rule
Only C–C bonds in backbone → addition polymerisation
Ester or amide links present → condensation polymerisation
Interpretation Strategy
- Look along the polymer backbone
- Identify any –COO– or –CONH– links
- If present → condensation polymerisation
- If absent and only C–C bonds remain → addition polymerisation
Condensation polymers can always be identified by the presence of ester or amide linkages in the polymer chain.
Example
A polymer contains the repeating section –CH₂–CH(CH₃)–. Deduce the type of polymerisation involved.
▶️ Answer / Explanation
The polymer backbone contains only carbon–carbon single bonds.
It must have been formed by addition polymerisation.
Example
A polymer segment contains the linkage –NH–(CH₂)₆–NHCO–(CH₂)₄–CO–. Deduce the type of polymerisation involved.
▶️ Answer / Explanation
The polymer contains amide (–CONH–) linkages.
This indicates condensation polymerisation with elimination of a small molecule.