CIE AS/A Level Chemistry 35.3 Degradable polymers Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 35.3 Degradable polymers Study Notes – New Syllabus
CIE AS/A Level Chemistry 35.3 Degradable polymers Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
recognise that poly(alkenes) are chemically inert and can therefore be difficult to biodegrade
recognise that some polymers can be degraded by the action of light
recognise that polyesters and polyamides are biodegradable by acidic and alkaline hydrolysis
Chemical Inertness of Poly(alkenes)
Poly(alkenes) (e.g. poly(ethene), poly(propene)) are widely used plastics. You must be able to recognise that they are chemically inert and explain why this makes them difficult to biodegrade.
Structure of Poly(alkenes)
Poly(alkenes) are formed by addition polymerisation of alkenes.
![]()
Their polymer chains consist of:
- only C–C and C–H bonds
- no functional groups (e.g. –COO– or –CONH–)
Why Poly(alkenes) Are Chemically Inert
C–C and C–H bonds are strong and non-polar.
As a result:
- poly(alkenes) do not react readily with acids or alkalis
- they are resistant to oxidation and hydrolysis
- they are not easily attacked by enzymes
Biodegradation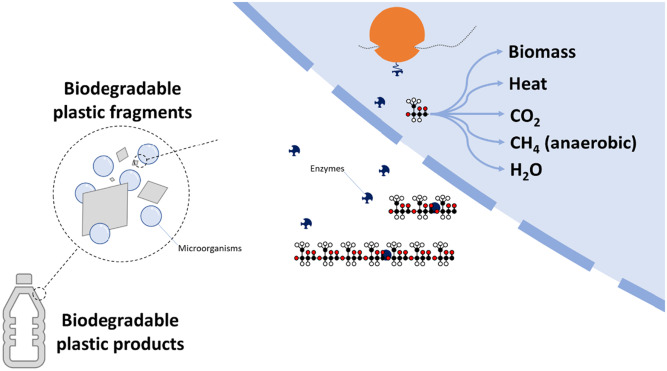
Biodegradation relies on enzymes breaking chemical bonds.
Enzymes typically act on polar functional groups such as:
- esters (–COO–)
- amides (–CONH–)
Because poly(alkenes) do not contain these groups, enzymes cannot bind to or break down the polymer chains effectively.
Environmental Consequence
Poly(alkenes) are therefore:
- non-biodegradable or very slow to biodegrade
- persistent in the environment
- a major contributor to plastic pollution
Poly(alkenes) are chemically inert because they contain only strong C–C and C–H bonds, so they are difficult to biodegrade.
Example
Explain why poly(ethene) does not readily biodegrade.
▶️ Answer / Explanation
Poly(ethene) contains only strong C–C and C–H bonds and has no functional groups.
As a result, it is chemically inert and cannot be easily broken down by enzymes.
Example
Compare the biodegradability of a poly(alkene) and a polyester.
▶️ Answer / Explanation
Poly(alkenes) contain only C–C and C–H bonds and are chemically inert, so they are difficult to biodegrade.
Polyesters contain ester linkages that can be hydrolysed, making them more biodegradable.
Degradation of Polymers by Light
Some polymers can be degraded by the action of light, particularly ultraviolet (UV) radiation. You must be able to recognise that this process can break polymer chains and change the properties of the material.
Photodegradation
Degradation of polymers by light is known as photodegradation.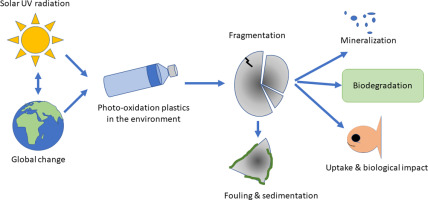
UV light has sufficient energy to break chemical bonds within polymer chains.
How Light Causes Degradation
When UV light is absorbed by a polymer:
- C–C or C–H bonds can break
- polymer chains become shorter
- the polymer becomes weaker and more brittle
Oxygen is often involved, leading to photo-oxidation.
Polymers Affected by Light
Many common plastics, such as poly(alkenes), can be degraded by UV light.
This is why plastics exposed to sunlight may:
- discolour
- crack or become brittle
- lose mechanical strength
Use of UV Stabilising Additives
To reduce light-induced degradation, polymers often contain:
- UV absorbers
- antioxidants
These additives increase the lifetime of plastics used outdoors.
Environmental Significance
Photodegradation can help break plastics into smaller fragments.
However, this does not necessarily mean full biodegradation, and can contribute to the formation of microplastics.
Example
Explain why a plastic left outdoors becomes brittle over time.
▶️ Answer / Explanation
UV light from sunlight breaks bonds in the polymer chains.
This shortens the chains and weakens the material, making it brittle.
Example
State one advantage and one disadvantage of polymer degradation by light.
▶️ Answer / Explanation
Advantage: sunlight can help reduce the lifetime of some plastics.
Disadvantage: degradation can produce microplastics rather than complete biodegradation.
Biodegradability of Polyesters and Polyamides
Polyesters and polyamides are examples of condensation polymers. You must be able to recognise that these polymers can be biodegraded by acidic or alkaline hydrolysis.
Key Structural Feature
Both polyesters and polyamides contain polar functional groups in their polymer backbone:
- polyesters → ester linkages, –COO–
- polyamides → amide linkages, –CONH–
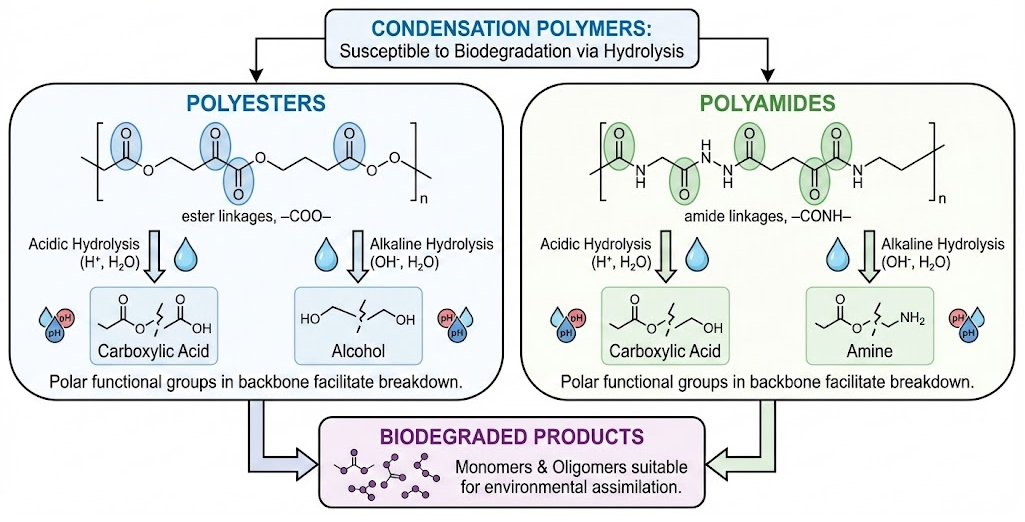
These linkages can be broken by hydrolysis.
Hydrolysis of Polyesters
Polyesters are biodegradable because their ester bonds can be hydrolysed.
Under acidic or alkaline conditions:
- ester linkages are broken
- the polymer chain is split into shorter fragments
- eventually forming diols and carboxylic acids (or carboxylate salts)
This makes polyesters susceptible to chemical and biological breakdown.
Hydrolysis of Polyamides
Polyamides are also biodegradable, although generally more resistant than polyesters.
Under acidic or alkaline hydrolysis:
- amide (peptide) bonds are broken
- polymer chains break down into amino acids or amines and carboxylic acids
This is the basis of protein digestion in biological systems.
Why This Leads to Biodegradability
Enzymes and environmental conditions can promote acidic or alkaline hydrolysis.
Because polyesters and polyamides contain hydrolysable linkages, they:
- are less chemically inert than poly(alkenes)
- can be broken down over time
- are considered biodegradable
Polyesters and polyamides are biodegradable because their ester and amide linkages can be broken by acidic or alkaline hydrolysis.
Example
State why polyesters are more biodegradable than poly(alkenes).
▶️ Answer / Explanation
Polyesters contain ester linkages that can be hydrolysed by acids or alkalis.
Poly(alkenes) contain only C–C and C–H bonds, which are resistant to hydrolysis.
Example
Explain why both polyesters and polyamides can be broken down in biological systems.
▶️ Answer / Explanation
Both polymers contain polar ester or amide linkages.
These linkages can be hydrolysed under acidic or alkaline conditions, including enzyme-catalysed reactions.
As a result, the polymer chains can be broken down into smaller molecules.
