CIE AS/A Level Chemistry 37.1 Thin-layer chromatography Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 37.1 Thin-layer chromatography Study Notes – New Syllabus
CIE AS/A Level Chemistry 37.1 Thin-layer chromatography Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe and understand the terms:
(a) stationary phase, for example aluminium oxide (on a solid support)
(b) mobile phase; a polar or non-polar solvent
(c) Rf value
(d) solvent front and baselineinterpret Rf values
explain the differences in Rf values in terms of interaction with the stationary phase and of relative
solubility in the mobile phase
Key Terms Used in Chromatography
Chromatography is a technique used to separate and identify components of a mixture. You must be able to describe and understand the following key terms.
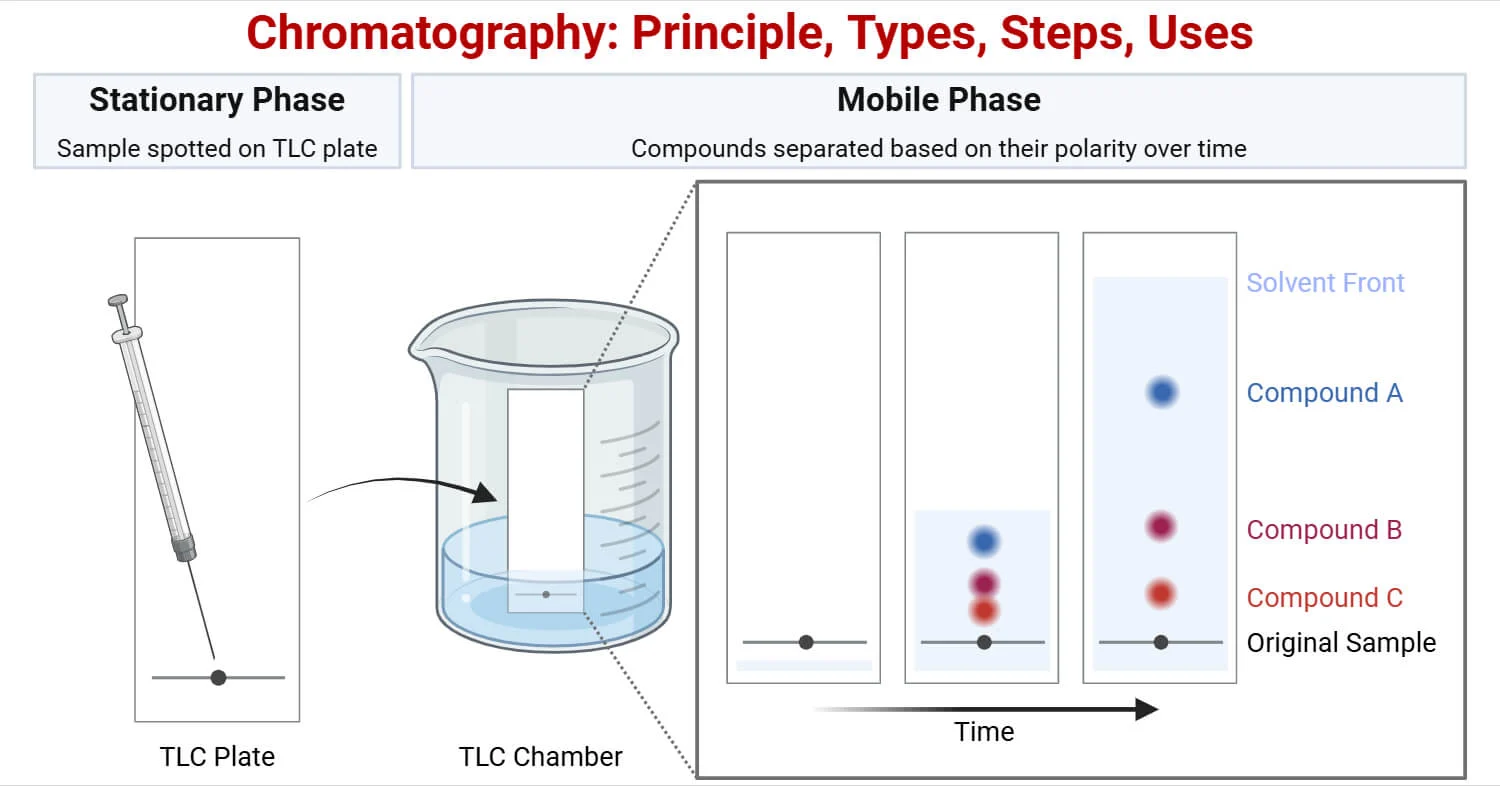
Stationary Phase
The stationary phase is the material that does not move during chromatography.
It is usually a solid or a liquid supported on a solid.
- Common example: aluminium oxide on a solid support
- The stationary phase is usually polar
Substances that interact more strongly with the stationary phase move more slowly.
Mobile Phase
The mobile phase is the liquid or gas that moves through the stationary phase.
In chromatography, the mobile phase is a solvent, which may be:
- polar (e.g. ethanol)
- non-polar (e.g. hexane)
Substances that dissolve more readily in the mobile phase travel further.![]()
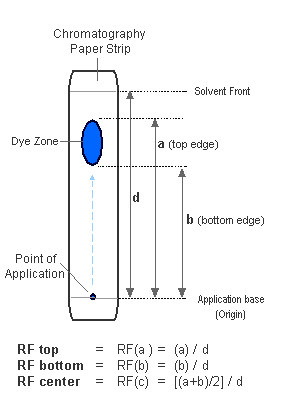
Rf Value
The Rf value (retention factor) is a measure of how far a substance travels relative to the solvent.
It is calculated using the equation:
\( \mathrm{Rf = \dfrac{\text{distance travelled by substance}}{\text{distance travelled by solvent front}}} \)
Rf values have no units and always lie between 0 and 1.
Solvent Front and Baseline
The baseline is the line drawn near the bottom of the chromatography paper or plate.
The sample spots are placed on the baseline at the start of the experiment.
The solvent front is the furthest point reached by the solvent as it moves up the stationary phase.
Distances used in Rf calculations are measured from the baseline.
Example
A compound travels 4.2 cm from the baseline. The solvent front travels 6.0 cm. Calculate the Rf value of the compound.
▶️ Answer / Explanation
Use the Rf equation:
\( \mathrm{Rf = \dfrac{4.2}{6.0}} \)
\( \mathrm{Rf = 0.70} \)
The Rf value of the compound is 0.70.
Example
Two compounds are separated using chromatography with aluminium oxide as the stationary phase. Compound A moves much further than compound B. Explain this observation.
▶️ Answer / Explanation
Aluminium oxide is a polar stationary phase.
Compound B interacts more strongly with the stationary phase and is retained for longer.
Compound A interacts less strongly with the stationary phase and is more soluble in the mobile phase.
As a result, compound A travels further up the chromatogram.
Interpreting Rf Values
Rf values are used to interpret chromatograms and help identify substances. You must be able to use Rf values to make comparisons and draw conclusions about a compound’s behaviour.
What an Rf Value Shows
An Rf value indicates how far a substance travels relative to the solvent front. It reflects the balance of attraction between the stationary phase and the mobile phase.
![]()
Interpreting Size of Rf
- High Rf: substance moves further up the plate
- Low Rf: substance stays closer to the baseline
With a polar stationary phase (e.g. aluminium oxide):
- More polar substances have lower Rf values
- Less polar substances have higher Rf values
Using Rf Values to Identify Compounds
Rf values can be used to identify a compound by comparison with known values, but only if the experimental conditions are the same.
Valid comparisons require the same:
- stationary phase
- mobile phase
- temperature
If two substances have the same Rf value under identical conditions, they are likely to be the same compound.
Limitations
- An Rf value cannot prove identity on its own
- Different compounds can occasionally have similar Rf values
Example
A compound has an Rf value of 0.82 when aluminium oxide is used as the stationary phase. Another compound has an Rf value of 0.35 under the same conditions. State which compound is more polar and explain your answer.
▶️ Answer / Explanation
The compound with Rf = 0.35 is more polar.
Polar substances interact more strongly with the polar stationary phase.
This causes them to move more slowly and have lower Rf values.
Example
A student compares a pure sample of compound X with a reaction mixture. Both are run on the same TLC plate using the same solvent.
The reaction mixture produces two spots, one of which has the same Rf value as compound X. Interpret these results.
▶️ Answer / Explanation
The spot with the same Rf value as compound X indicates that compound X is present in the mixture.
The second spot shows that at least one other substance is also present.
This suggests that the reaction is incomplete or that a by-product has formed.
Explaining Differences in Rf Values
Differences in Rf values arise because substances interact differently with the stationary phase and have different relative solubilities in the mobile phase.
Interaction with the Stationary Phase
The stationary phase (e.g. aluminium oxide) is usually polar. Substances that interact strongly with it are held more tightly and move more slowly.
- Stronger interaction with the stationary phase → lower Rf
- Weaker interaction with the stationary phase → higher Rf
More polar substances form stronger intermolecular attractions (e.g. hydrogen bonding, dipole–dipole interactions) with a polar stationary phase, so they travel a shorter distance.
Relative Solubility in the Mobile Phase
The mobile phase is a solvent which may be polar or non-polar. Substances that are more soluble in the mobile phase are carried further up the plate.
- Greater solubility in the mobile phase → higher Rf
- Lower solubility in the mobile phase → lower Rf
A substance with high solubility in the mobile phase spends more time moving with the solvent and less time adsorbed onto the stationary phase.
Overall Explanation
The Rf value depends on the competition between attraction to the stationary phase and solubility in the mobile phase.
- Strong stationary-phase interactions and low mobile-phase solubility → low Rf
- Weak stationary-phase interactions and high mobile-phase solubility → high Rf
Example
Two compounds are separated using aluminium oxide as the stationary phase. Compound A has an Rf value of 0.28 and compound B has an Rf value of 0.76. Explain the difference in their Rf values.
▶️ Answer / Explanation
Aluminium oxide is a polar stationary phase.
Compound A interacts more strongly with the stationary phase, so it is held more tightly and moves a shorter distance.
Compound B interacts more weakly with the stationary phase and is more soluble in the mobile phase, so it travels further and has a higher Rf value.
Example
A polar stationary phase and a non-polar mobile phase are used to separate three compounds. Compound X has the lowest Rf value, compound Y has an intermediate Rf value, and compound Z has the highest Rf value.
Explain these results in terms of both stationary-phase interactions and relative solubility.
▶️ Answer / Explanation
Compound X is the most polar and interacts most strongly with the polar stationary phase, so it is retained for longest and has the lowest Rf value.
Compound Y has intermediate polarity, so it shows moderate interaction with the stationary phase and moderate solubility in the mobile phase.
Compound Z is the least polar, interacts weakly with the stationary phase, and is most soluble in the non-polar mobile phase.
As a result, compound Z travels the furthest and has the highest Rf value.
