CIE AS/A Level Chemistry 37.2 Gas / liquid chromatography Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 37.2 Gas / liquid chromatography Study Notes – New Syllabus
CIE AS/A Level Chemistry 37.2 Gas / liquid chromatography Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe and understand the terms:
(a) stationary phase; a high boiling point non-polar liquid (on a solid support)
(b) mobile phase; an unreactive gas
(c) retention timeinterpret gas/liquid chromatograms in terms of the percentage composition of a mixture
explain retention times in terms of interaction with the stationary phase
Key Terms Used in Gas Chromatography (GC)
Gas chromatography is used to separate and identify volatile compounds. You must be able to describe and understand the following key terms.
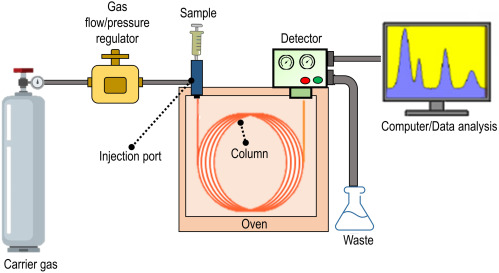
Stationary Phase
In gas chromatography, the stationary phase is a high boiling point non-polar liquid that is coated onto a solid support inside the column.
The stationary phase does not vaporise during the experiment due to its high boiling point.
Substances that interact more strongly with the stationary phase spend more time adsorbed and therefore move more slowly through the column.
Mobile Phase
The mobile phase in gas chromatography is an unreactive gas, known as the carrier gas.
Common carrier gases include helium and nitrogen.
The mobile phase carries the vaporised sample through the column but does not react with any of the substances being analysed.
Retention Time
The retention time is the time taken for a substance to pass through the column from injection to detection.
Each compound has a characteristic retention time under a given set of conditions.
Substances with stronger interactions with the stationary phase or higher boiling points generally have longer retention times.
Example
Two compounds are analysed by gas chromatography using the same column. Compound A has a shorter retention time than compound B.
Explain this observation.
▶️ Answer / Explanation
Compound A interacts less strongly with the non-polar stationary phase.
It therefore spends less time in the stationary phase and is carried more quickly by the mobile phase.
This results in a shorter retention time for compound A.
Example
A mixture of two hydrocarbons is separated by gas chromatography. One hydrocarbon has a significantly longer retention time than the other.
Explain this difference in terms of both volatility and interaction with the stationary phase.
▶️ Answer / Explanation
The hydrocarbon with the longer retention time has a higher boiling point, so it is less volatile.
It also experiences stronger intermolecular attractions with the non-polar stationary phase.
As a result, it spends more time in the stationary phase and takes longer to pass through the column.
Interpreting Gas–Liquid Chromatograms: Percentage Composition
Gas–liquid chromatography (GLC) can be used to determine the percentage composition of a mixture by analysing the peaks shown on a chromatogram.
Chromatogram Peaks
In a gas–liquid chromatogram, each substance in the mixture produces a separate peak.
- The position of a peak shows the retention time
- The area under a peak is proportional to the amount of that substance
Peak height alone is not reliable; it is the peak area that is used to calculate composition.
Calculating Percentage Composition
The percentage composition of a component is found using:
\( \mathrm{\%\ composition = \dfrac{\text{area of one peak}}{\text{total area of all peaks}} \times 100} \)
All peak areas must be measured under the same experimental conditions.
Interpreting Results
- Larger peak area → greater proportion of that component
- Smaller peak area → smaller proportion of that component
- Total percentage of all components ≈ 100%
Example
A gas–liquid chromatogram of a mixture shows two peaks. The areas under the peaks are 30 units and 70 units.
Calculate the percentage composition of each component.
▶️ Answer / Explanation
Total peak area = \( \mathrm{30 + 70 = 100} \).
Percentage of component 1:
\( \mathrm{\dfrac{30}{100} \times 100 = 30\%} \)
Percentage of component 2:
\( \mathrm{\dfrac{70}{100} \times 100 = 70\%} \)
Example
A mixture is analysed by gas–liquid chromatography and produces three peaks with areas of 12.5, 37.5 and 50.0 units.
(a) Calculate the percentage composition of each component.
(b) State which component is present in the greatest amount.
▶️ Answer / Explanation
Total peak area = \( \mathrm{12.5 + 37.5 + 50.0 = 100.0} \).
Percentages:
\( \mathrm{\dfrac{12.5}{100.0} \times 100 = 12.5\%} \)
\( \mathrm{\dfrac{37.5}{100.0} \times 100 = 37.5\%} \)
\( \mathrm{\dfrac{50.0}{100.0} \times 100 = 50.0\%} \)
The component with a peak area of 50.0 units is present in the greatest amount.
Explaining Retention Times in Gas Chromatography
In gas chromatography, different substances have different retention times. These differences can be explained by the strength of interaction between each substance and the stationary phase.
Interaction with the Stationary Phase
The stationary phase in gas chromatography is a high boiling point, non-polar liquid coated onto a solid support.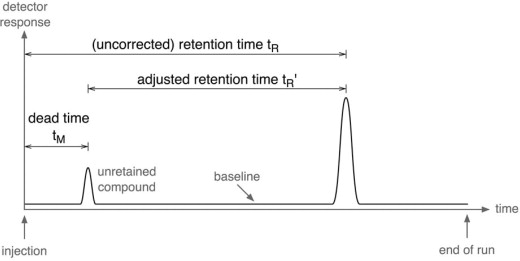
As substances pass through the column, they repeatedly:
- dissolve in the stationary phase
- evaporate back into the mobile phase
Substances that interact more strongly with the stationary phase spend more time dissolved in it.
Effect on Retention Time
- Stronger interaction with stationary phase → longer retention time
- Weaker interaction with stationary phase → shorter retention time
Stronger interactions are usually due to:
- greater polarity (even in mainly non-polar systems)
- larger surface area or higher molar mass
- higher boiling point
Overall Explanation
Retention time is determined by how strongly a substance is attracted to the stationary phase. The more time it spends interacting with the stationary phase, the longer it takes to pass through the column.
Example
Two organic liquids are analysed using gas chromatography with the same column. Compound A has a shorter retention time than compound B. Explain this difference.
▶️ Answer / Explanation
Compound A interacts less strongly with the stationary phase.
It therefore spends less time dissolved in the stationary phase and moves more quickly with the mobile phase.
As a result, compound A has a shorter retention time.
Example
A mixture of hydrocarbons is analysed by gas chromatography. One hydrocarbon has a much longer retention time than the others.
Explain why this hydrocarbon has the longest retention time.
▶️ Answer / Explanation
The hydrocarbon with the longest retention time has the strongest intermolecular attractions with the stationary phase.
It also has a higher boiling point and is less volatile.
As a result, it spends more time dissolved in the stationary phase and takes longer to pass through the column.
