CIE AS/A Level Chemistry 7.2 Brønsted–Lowry theory of acids and bases Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 7.2 Brønsted–Lowry theory of acids and bases Study Notes – New Syllabus
CIE AS/A Level Chemistry 7.2 Brønsted–Lowry theory of acids and bases Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
state the names and formulas of common acids:
hydrochloric acid – HCl
sulfuric acid – H₂SO₄
nitric acid – HNO₃
ethanoic acid – CH₃COOHstate the names and formulas of common alkalis:
sodium hydroxide – NaOH
potassium hydroxide – KOH
ammonia – NH₃describe the Brønsted–Lowry theory of acids and bases
describe strong acids and bases as fully dissociated in aqueous solution and weak acids and bases as partially dissociated
appreciate that pure water has pH 7, acidic solutions pH below 7 and alkaline solutions pH above 7
explain qualitatively differences between strong and weak acids, including reaction with reactive metals and differences in pH using indicators or conductivity
understand that neutralisation occurs when H⁺(aq) and OH⁻(aq) form H₂O(l)
understand that salts are formed in neutralisation reactions
sketch pH titration curves for combinations of strong and weak acids with strong and weak alkalis
select suitable indicators for acid–alkali titrations (pKa values will not be used)
Common Acids: Names and Formulae
Acids are substances that produce hydrogen ions, \( \mathrm{H^+} \), in aqueous solution. At this level, you are required to recall the names and chemical formulae of a limited number of common acids.
List of Common Acids
| Acid name | Chemical formula | Type of acid |
|---|---|---|
| Hydrochloric acid | \( \mathrm{HCl} \) | Strong acid |
| Sulfuric acid | \( \mathrm{H_2SO_4} \) | Strong diprotic acid |
| Nitric acid | \( \mathrm{HNO_3} \) | Strong acid |
| Ethanoic acid | \( \mathrm{CH_3COOH} \) | Weak acid |
Example
State the formula of nitric acid.
▶️ Answer / Explanation
The formula of nitric acid is \( \mathrm{HNO_3} \).
Example
Name the acid with the formula \( \mathrm{H_2SO_4} \).
▶️ Answer / Explanation
The acid is sulfuric acid.
Example
Identify which of the following acids is weak: \( \mathrm{HCl} \), \( \mathrm{HNO_3} \), \( \mathrm{CH_3COOH} \).
▶️ Answer / Explanation
Ethanoic acid, \( \mathrm{CH_3COOH} \), is a weak acid.
Common Alkalis: Names and Formulae
Alkalis are bases that dissolve in water to produce hydroxide ions, \( \mathrm{OH^-} \). At this level, you are required to recall the names and chemical formulae of a limited number of common alkalis.
List of Common Alkalis
| Alkali name | Chemical formula | Type of alkali |
|---|---|---|
| Sodium hydroxide | \( \mathrm{NaOH} \) | Strong alkali |
| Potassium hydroxide | \( \mathrm{KOH} \) | Strong alkali |
| Ammonia | \( \mathrm{NH_3} \) | Weak alkali |
Example
State the formula of sodium hydroxide.
▶️ Answer / Explanation
The formula of sodium hydroxide is \( \mathrm{NaOH} \).
Example
Name the alkali with the formula \( \mathrm{KOH} \).
▶️ Answer / Explanation
The alkali is potassium hydroxide.
Example
Identify which of the following alkalis is weak: \( \mathrm{NaOH} \), \( \mathrm{KOH} \), \( \mathrm{NH_3} \).
▶️ Answer / Explanation
Ammonia, \( \mathrm{NH_3} \), is a weak alkali.
Brønsted–Lowry Theory of Acids and Bases
The Brønsted–Lowry theory provides a simple and widely used definition of acids and bases based on the transfer of protons. This theory applies to reactions in aqueous solution and beyond.
- A Brønsted–Lowry acid is a species that donates a proton, \( \mathrm{H^+} \).
- A Brønsted–Lowry base is a species that accepts a proton, \( \mathrm{H^+} \).
Acid–base reactions involve the transfer of a proton from the acid to the base.
Acid–Base Reactions
In a Brønsted–Lowry acid–base reaction:

- The acid loses a proton.
- The base gains a proton.
- Both processes occur simultaneously.
Example: \( \mathrm{HCl + H_2O \rightarrow H_3O^+ + Cl^-} \)
Here, HCl donates a proton and acts as an acid, while water accepts a proton and acts as a base.
Conjugate Acid–Base Pairs
A conjugate acid–base pair consists of two species that differ by one proton.
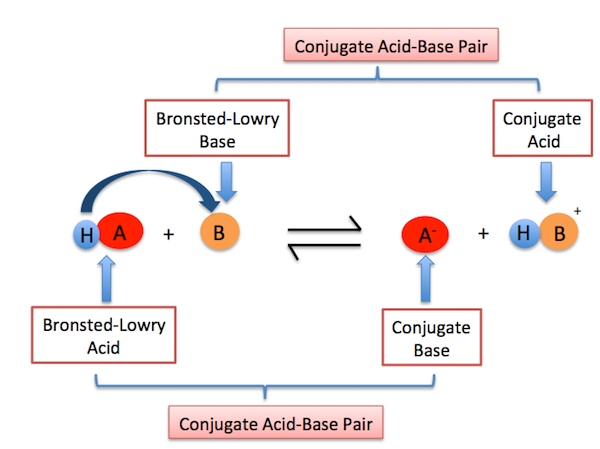
- An acid forms its conjugate base after donating a proton.
- A base forms its conjugate acid after accepting a proton.
Example: \( \mathrm{NH_3 + H_2O \rightleftharpoons NH_4^+ + OH^-} \)
Conjugate pairs are:
- \( \mathrm{NH_3 / NH_4^+} \)
- \( \mathrm{H_2O / OH^-} \)
Amphoteric Species
Some substances can act as both acids and bases. These are described as amphoteric.
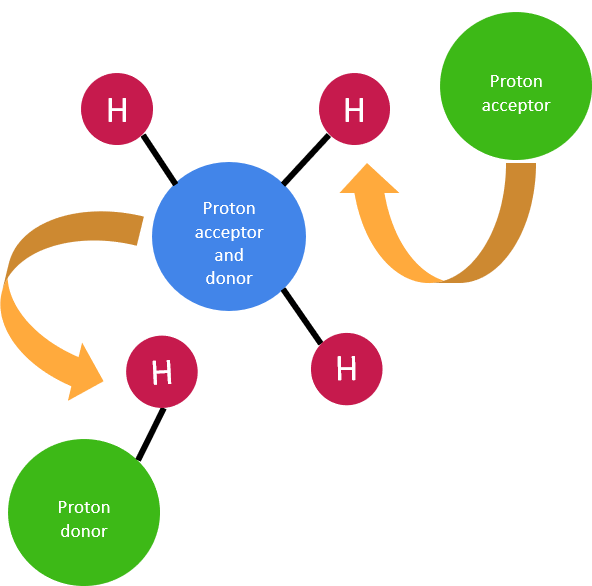
- Water is a common amphoteric substance.
- It can donate or accept a proton depending on the reaction.
Example: As an acid: \( \mathrm{H_2O \rightarrow H^+ + OH^-} \) As a base: \( \mathrm{H_2O + H^+ \rightarrow H_3O^+} \)
Example
Define a Brønsted–Lowry acid.
▶️ Answer / Explanation
A Brønsted–Lowry acid is a proton donor.
Example
Identify the acid and base in the reaction:
\( \mathrm{HNO_3 + H_2O \rightarrow H_3O^+ + NO_3^-} \)
▶️ Answer / Explanation
HNO₃ donates a proton and is the acid.
Water accepts a proton and is the base.
Example
Explain why ammonia acts as a base according to the Brønsted–Lowry theory.
▶️ Answer / Explanation
Ammonia accepts a proton from water to form \( \mathrm{NH_4^+} \).
Therefore, ammonia is a Brønsted–Lowry base.
Strong and Weak Acids and Bases

Acids and bases can be classified as strong or weak based on the extent to which they dissociate in aqueous solution. This description refers to the degree of ionisation, not the concentration of the solution.
Strong Acids
A strong acid is one that is fully dissociated in aqueous solution.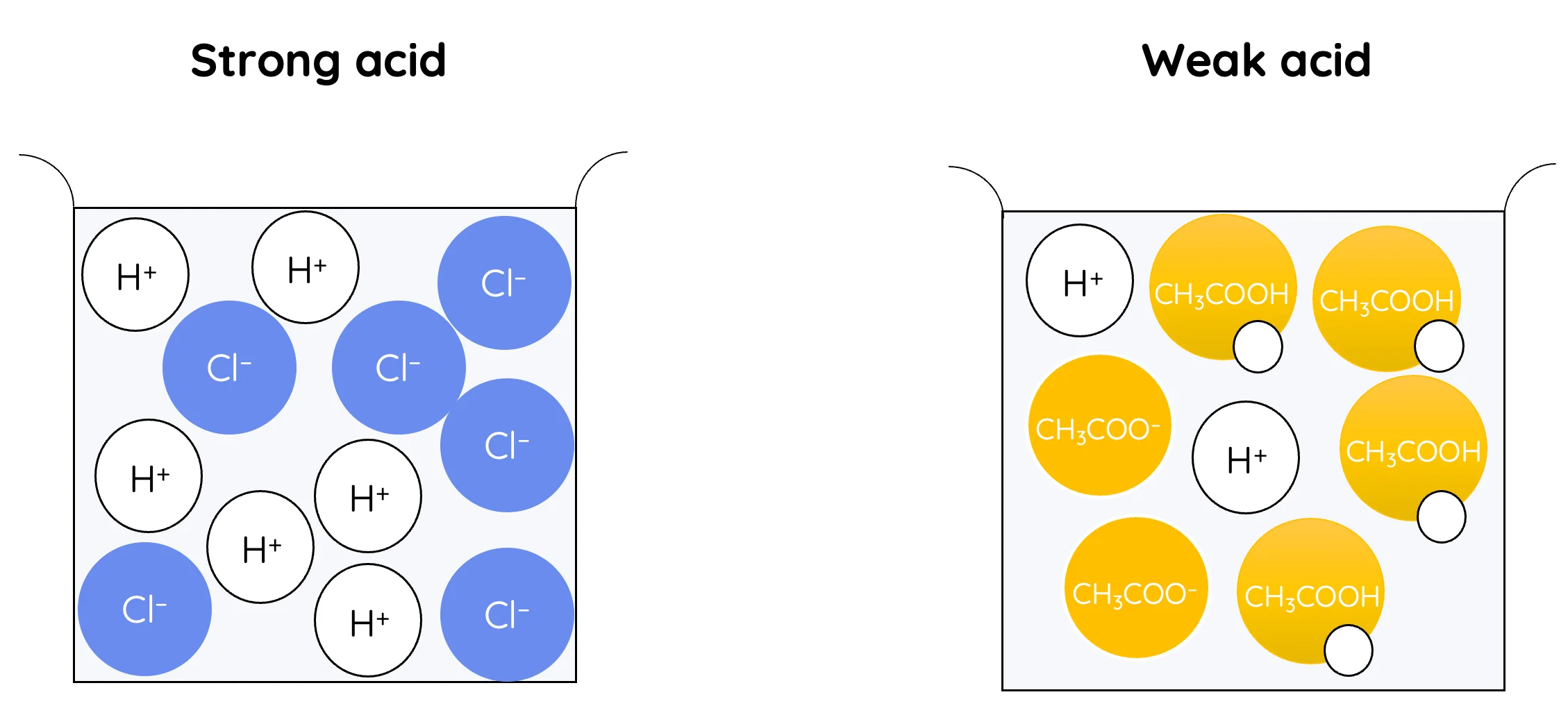
Example: \( \mathrm{HCl(aq) \rightarrow H^+(aq) + Cl^-(aq)} \)
- Almost all acid molecules ionise.
- The concentration of \( \mathrm{H^+} \) ions is high.
- Examples include hydrochloric acid, nitric acid and sulfuric acid.
Weak Acids
A weak acid is one that is partially dissociated in aqueous solution.
Example: \( \mathrm{CH_3COOH(aq) \rightleftharpoons H^+(aq) + CH_3COO^-(aq)} \)
- Only a small fraction of acid molecules ionise.
- Most molecules remain undissociated.
- Ethanoic acid is a common weak acid.
Strong Bases (Alkalis)
A strong base (alkali) is one that is fully dissociated in aqueous solution.
Example: \( \mathrm{NaOH(aq) \rightarrow Na^+(aq) + OH^-(aq)} \)
- Hydroxide ions are produced completely.
- Examples include sodium hydroxide and potassium hydroxide.
Weak Bases (Alkalis)
A weak base is one that is partially dissociated in aqueous solution.
Example: \( \mathrm{NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)} \)
- Only some ammonia molecules form hydroxide ions.
- Most ammonia molecules remain unchanged.
- Ammonia is a weak base.
Key Differences
| Type | Degree of dissociation in water | Example |
|---|---|---|
| Strong acid | Fully dissociated | \( \mathrm{HCl} \) |
| Weak acid | Partially dissociated | \( \mathrm{CH_3COOH} \) |
| Strong base | Fully dissociated | \( \mathrm{NaOH} \) |
| Weak base | Partially dissociated | \( \mathrm{NH_3} \) |
Example
State whether hydrochloric acid is strong or weak.
▶️ Answer / Explanation
Hydrochloric acid is a strong acid because it fully dissociates in water.
Example
Explain why ethanoic acid is described as a weak acid.
▶️ Answer / Explanation
Only a small proportion of ethanoic acid molecules dissociate in aqueous solution.
Example
Write an equation to show that ammonia is a weak base.
▶️ Answer / Explanation
\( \mathrm{NH_3(aq) + H_2O(l) \rightleftharpoons NH_4^+(aq) + OH^-(aq)} \)
pH Scale: Acids, Alkalis and Neutral Solutions
The pH scale is used to describe how acidic or alkaline a solution is. It is based on the concentration of hydrogen ions, \( \mathrm{H^+} \), in aqueous solution.
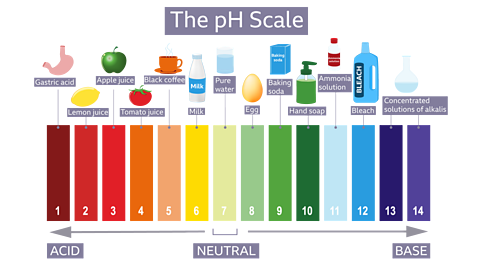
pH of Water
Pure water at room temperature is neutral.
pH of pure water = 7
In neutral water, the concentrations of hydrogen ions and hydroxide ions are equal.
Acidic Solutions
An acidic solution has a pH value below 7.
- Acid solutions contain a higher concentration of \( \mathrm{H^+} \) ions than water.
- The lower the pH, the more acidic the solution.
- Strong acids have very low pH values.
Example: Hydrochloric acid typically has a pH of about 1–2.
Alkaline Solutions
An alkaline solution has a pH value above 7.
- Alkaline solutions contain a higher concentration of \( \mathrm{OH^-} \) ions.
- The higher the pH, the more alkaline the solution.
- Strong alkalis have very high pH values.
Example: Sodium hydroxide solution typically has a pH of about 13–14.
pH Values
| Type of solution | pH value | Example |
|---|---|---|
| Acidic | Below 7 | \( \mathrm{HCl(aq)} \) |
| Neutral | 7 | Pure water |
| Alkaline | Above 7 | \( \mathrm{NaOH(aq)} \) |
Example
State the pH of pure water.
▶️ Answer / Explanation
The pH of pure water is 7.
Example
State whether a solution with pH 4 is acidic, neutral or alkaline.
▶️ Answer / Explanation
A pH of 4 is below 7, so the solution is acidic.
Example
A solution has a pH of 12. Describe the nature of this solution and compare it to pure water.
▶️ Answer / Explanation
The solution is alkaline because its pH is greater than 7.
It contains a higher concentration of \( \mathrm{OH^-} \) ions and fewer \( \mathrm{H^+} \) ions than pure water.
Differences in Behaviour Between Strong and Weak Acids
Strong and weak acids differ in how completely they dissociate in aqueous solution. These differences explain their different behaviour in reactions, pH values, and electrical conductivity.
Extent of Dissociation
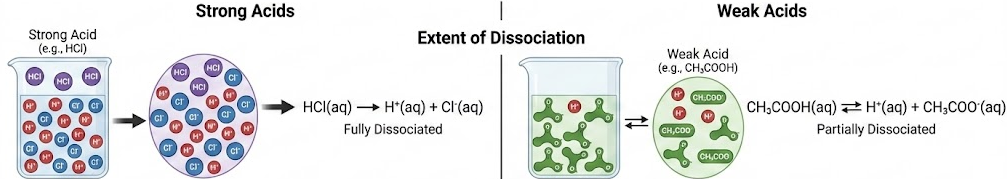
A strong acid is fully dissociated in aqueous solution, producing a high concentration of hydrogen ions.
Example: \( \mathrm{HCl(aq) \rightarrow H^+(aq) + Cl^-(aq)} \)
A weak acid is only partially dissociated in aqueous solution.
Example: \( \mathrm{CH_3COOH(aq) \rightleftharpoons H^+(aq) + CH_3COO^-(aq)} \)
Reaction with a Reactive Metal
Both strong and weak acids react with reactive metals (such as magnesium or zinc) to produce hydrogen gas.

General reaction: \( \mathrm{acid + metal \rightarrow salt + H_2} \)
- Strong acids react more vigorously because they contain a higher concentration of \( \mathrm{H^+} \) ions.
- Weak acids react more slowly because fewer \( \mathrm{H^+} \) ions are available.
Example comparison with magnesium:
\( \mathrm{Mg + 2HCl \rightarrow MgCl_2 + H_2} \) (fast, vigorous)
\( \mathrm{Mg + 2CH_3COOH \rightarrow (CH_3COO)_2Mg + H_2} \) (slow, gentle)
Differences in pH Values

For solutions of the same concentration:
- Strong acids have lower pH values.
- Weak acids have higher pH values.
Reason: strong acids release more \( \mathrm{H^+} \) ions into solution.
Use of a pH Meter and Universal Indicator
- A pH meter gives an accurate numerical pH value.
- A universal indicator shows pH by a colour change.
At the same concentration:
- A strong acid gives a red colour and very low pH.
- A weak acid gives an orange or yellow colour and a higher pH.
Electrical Conductivity
Electrical conductivity depends on the number of mobile ions in solution.

- Strong acids conduct electricity well because they produce many ions.
- Weak acids conduct electricity poorly because they produce fewer ions.
| Property | Strong acid | Weak acid |
|---|---|---|
| Dissociation | Complete | Partial |
| Reaction with metal | Fast and vigorous | Slow |
| pH value | Lower | Higher |
| Conductivity | High | Low |
Example
State one reason why hydrochloric acid reacts faster with magnesium than ethanoic acid.
▶️ Answer / Explanation
Hydrochloric acid is fully dissociated and provides more \( \mathrm{H^+} \) ions.
Example
Two acids of equal concentration are tested with universal indicator. One turns red and the other turns orange. Identify which is stronger.
▶️ Answer / Explanation
The acid turning red is stronger because it has a lower pH.
Example
Explain why a strong acid has a higher electrical conductivity than a weak acid of the same concentration.
▶️ Answer / Explanation
A strong acid is fully dissociated, producing more mobile ions that carry electric charge.
Neutralisation Reactions
A neutralisation reaction occurs when an acid reacts with a base (alkali). The reaction involves hydrogen ions from the acid and hydroxide ions from the base.

Neutralisation occurs when:
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
Hydrogen ions from the acid combine with hydroxide ions from the base to form water.
Acid–Base Neutralisation
In a typical neutralisation reaction:
- The acid provides \( \mathrm{H^+} \) ions.
- The base provides \( \mathrm{OH^-} \) ions.
- Water is formed, and a salt is also produced.
Example (hydrochloric acid and sodium hydroxide):
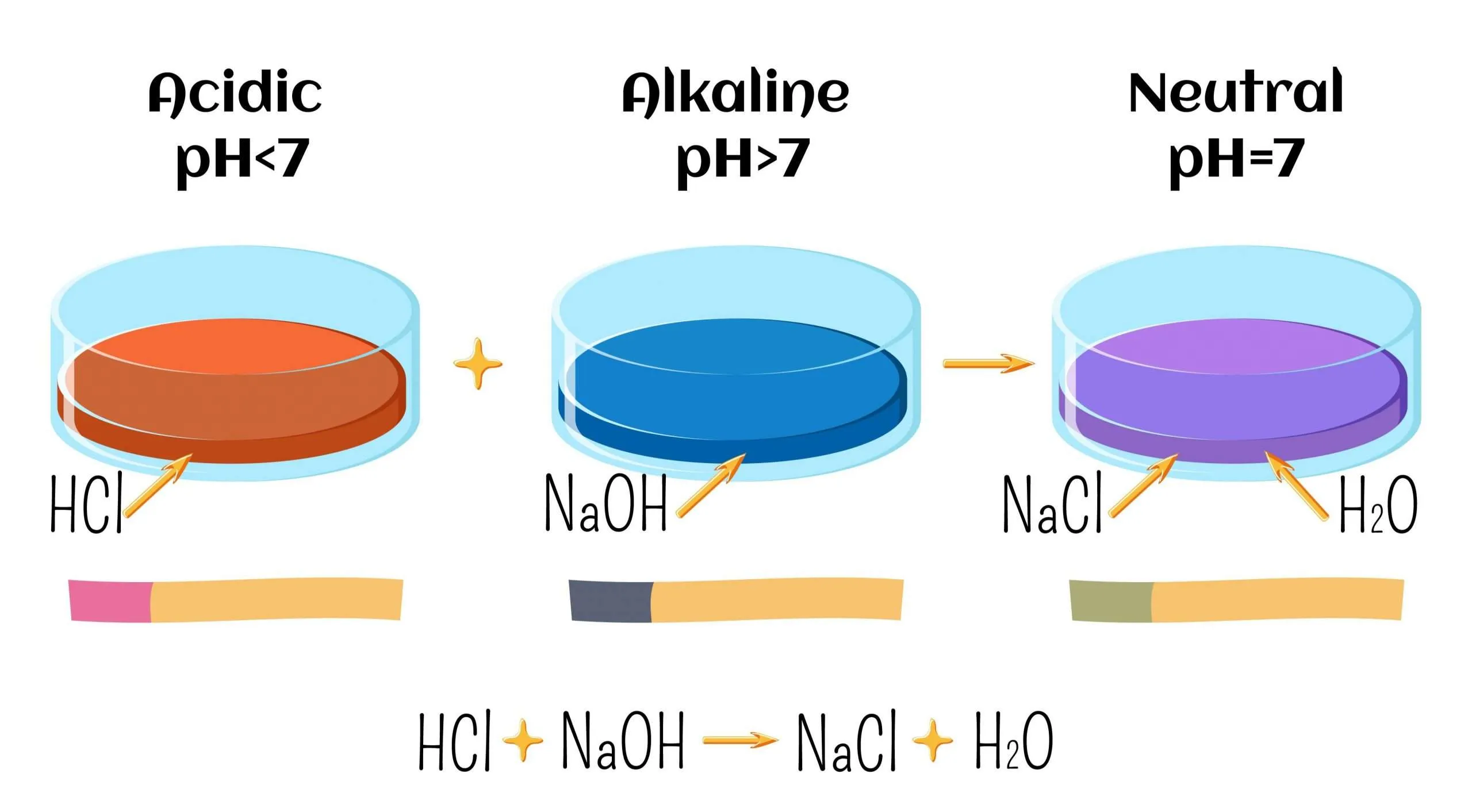
The net ionic equation for all neutralisation reactions between strong acids and strong bases is the same.
Net Ionic Equation
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
Spectator ions such as \( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) do not take part in the reaction and are omitted from the ionic equation.
Important Points
- Neutralisation reactions always produce water.
- The reaction reduces the acidity or alkalinity of the solution.
- If equal amounts of \( \mathrm{H^+} \) and \( \mathrm{OH^-} \) react, the solution becomes neutral.
Example
What two ions react together in a neutralisation reaction?
▶️ Answer / Explanation
\( \mathrm{H^+(aq)} \) ions and \( \mathrm{OH^-(aq)} \) ions react together.
Example
Write the ionic equation for the neutralisation of an acid with an alkali.
▶️ Answer / Explanation
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
Example
Explain why the same ionic equation applies to the neutralisation of hydrochloric acid by sodium hydroxide and nitric acid by potassium hydroxide.
▶️ Answer / Explanation
In both reactions, the reacting species are \( \mathrm{H^+} \) ions from the acid and \( \mathrm{OH^-} \) ions from the base.
The other ions are spectators and do not affect the neutralisation.
Formation of Salts in Neutralisation Reactions
In a neutralisation reaction, an acid reacts with a base (or alkali) to form a salt and water. This is a key feature of all neutralisation reactions.

A salt is an ionic compound formed when:
- the hydrogen ion \( \mathrm{H^+} \) from an acid is replaced by a metal ion or the ammonium ion
- the remaining part of the acid combines with the positive ion from the base
General Neutralisation Reaction
Acid + Base → Salt + Water
Water is formed from hydrogen ions and hydroxide ions:
\( \mathrm{H^+(aq) + OH^-(aq) \rightarrow H_2O(l)} \)
Examples of Salt Formation
1. Acid + Alkali
\( \mathrm{HCl(aq) + NaOH(aq) \rightarrow NaCl(aq) + H_2O(l)} \)
Salt formed: sodium chloride
2. Acid + Metal Oxide
\( \mathrm{H_2SO_4(aq) + CuO(s) \rightarrow CuSO_4(aq) + H_2O(l)} \)
Salt formed: copper(II) sulfate
3. Acid + Ammonia
\( \mathrm{HNO_3(aq) + NH_3(aq) \rightarrow NH_4NO_3(aq)} \)
Salt formed: ammonium nitrate
Important Points
- All neutralisation reactions produce a salt.
- The name of the salt depends on the acid and base used.
- Different acids form different types of salts:
- Hydrochloric acid → chlorides
- Sulfuric acid → sulfates
- Nitric acid → nitrates
Example
What two products are always formed in a neutralisation reaction?
▶️ Answer / Explanation
A salt and water are formed.
Example
State the salt formed when nitric acid reacts with potassium hydroxide.
▶️ Answer / Explanation
Potassium nitrate is formed.
Example
Explain how the salt ammonium sulfate is formed in a neutralisation reaction.
▶️ Answer / Explanation
Ammonium ions from ammonia combine with sulfate ions from sulfuric acid.
The hydrogen ions and hydroxide ions react to form water.
pH Titration Curves: Strong and Weak Acids with Strong and Weak Alkalis
A pH titration curve shows how the pH of a solution changes as a titrant is added. The shape of the curve depends on whether the acid and alkali are strong or weak.
![]()
Key Features Common to All Titration Curves
- The x-axis shows volume of titrant added.
- The y-axis shows pH.
- The equivalence point is where acid and base have reacted in exact stoichiometric amounts.
- The pH at the equivalence point depends on the strengths of the acid and base.
1. Strong Acid – Strong Alkali 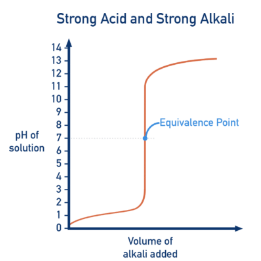
Example: hydrochloric acid titrated with sodium hydroxide.
- Initial pH is very low (≈ 1–2).
- Gradual rise in pH as alkali is added.
- Very steep vertical section near the equivalence point.
- Equivalence point at pH = 7.
- Final pH is very high (≈ 13–14).
This is because the salt formed does not affect pH.
2. Weak Acid – Strong Alkali
Example: ethanoic acid titrated with sodium hydroxide.
- Initial pH is moderately acidic (≈ 3–4).
- Presence of a buffer region where pH changes slowly.
- Less steep rise near equivalence point.
- Equivalence point above pH 7 (alkaline).
This occurs because the conjugate base formed reacts with water to produce \( \mathrm{OH^-} \).
3. Strong Acid – Weak Alkali
Example: hydrochloric acid titrated with ammonia.
- Initial pH is very low.
- Gradual increase in pH.
- Shorter and less steep vertical section.
- Equivalence point below pH 7 (acidic).
This is due to the acidic nature of the ammonium ion formed.
4. Weak Acid – Weak Alkali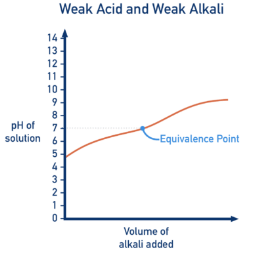
Example: ethanoic acid titrated with ammonia.
- Initial pH is moderately acidic.
- No sharp vertical section.
- Very gradual change in pH throughout.
- Equivalence point is poorly defined.
Because both acid and base are weak, the pH change is small.
| Titration | Initial pH | Equivalence point pH | Curve shape |
|---|---|---|---|
| Strong acid–strong alkali | Very low | 7 | Very steep |
| Weak acid–strong alkali | Moderate | > 7 | Steep, buffer region |
| Strong acid–weak alkali | Very low | < 7 | Less steep |
| Weak acid–weak alkali | Moderate | Poorly defined | Gradual |
Example
At what pH does the equivalence point occur for a strong acid–strong base titration?
▶️ Answer / Explanation
The equivalence point occurs at pH 7.
Example
Explain why the equivalence point is above pH 7 for a weak acid–strong alkali titration.
▶️ Answer / Explanation
The conjugate base of the weak acid reacts with water to produce hydroxide ions.
Example
Why is the equivalence point difficult to detect in a weak acid–weak alkali titration?
▶️ Answer / Explanation
Both the acid and base are weak, so the pH changes slowly and there is no sharp vertical section on the curve.
Selecting Suitable Indicators for Acid–Alkali Titrations
An indicator must change colour over the pH range where the titration curve shows a steep vertical section. A suitable indicator is one whose colour-change range lies entirely within this steep region.
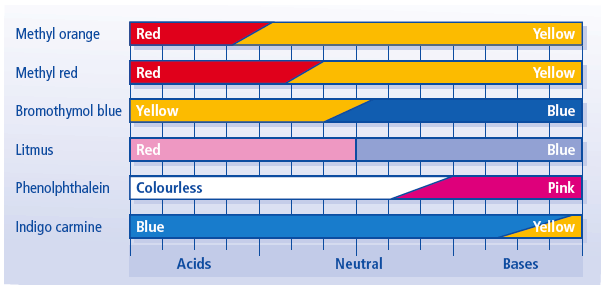
Key Principle: An indicator is suitable if its colour change occurs at the equivalence point.
This decision is made by looking at:
- the type of acid (strong or weak)
- the type of alkali (strong or weak)
- the pH at the equivalence point
At this level, pKa values are not required.
Common Indicators and Their pH Ranges
| Indicator | Colour change | Approximate pH range |
|---|---|---|
| Methyl orange | Red → Yellow | 3–4 |
| Methyl red | Red → Yellow | 4–6 |
| Phenolphthalein | Colourless → Pink | 8–10 |
Choice of Indicator for Different Titrations

1. Strong Acid – Strong Alkali
Suitable indicators: methyl orange, methyl red, phenolphthalein | 3. Strong Acid – Weak Alkali
Suitable indicator: methyl orange Unsuitable indicator: phenolphthalein |
2. Weak Acid – Strong Alkali
Suitable indicator: phenolphthalein Unsuitable indicator: methyl orange (changes too early) | 4. Weak Acid – Weak Alkali
|
| Titration type | Equivalence pH | Suitable indicator |
|---|---|---|
| Strong acid–strong alkali | 7 | Most indicators |
| Weak acid–strong alkali | > 7 | Phenolphthalein |
| Strong acid–weak alkali | < 7 | Methyl orange |
| Weak acid–weak alkali | Gradual change | None suitable |
Example
Which indicator is suitable for a strong acid–strong base titration?
▶️ Answer / Explanation
Either methyl orange or phenolphthalein is suitable.
Example
Choose a suitable indicator for titrating ethanoic acid with sodium hydroxide and explain your choice.
▶️ Answer / Explanation
Phenolphthalein is suitable because the equivalence point is above pH 7.
Example
Explain why no indicator is suitable for a weak acid–weak alkali titration.
▶️ Answer / Explanation
The pH changes gradually and there is no sharp equivalence point for a clear colour change.
