CIE AS/A Level Chemistry 8.2 Effect of temperature on reaction rates and the concept of activation energy Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 8.2 Effect of temperature on reaction rates and the concept of activation energy Study Notes – New Syllabus
CIE AS/A Level Chemistry 8.2 Effect of temperature on reaction rates and the concept of activation energy Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
define activation energy, Eₐ, as the minimum energy required for a collision to be effective
sketch and use the Boltzmann distribution to explain activation energy
explain qualitatively the effect of temperature on reaction rate using the Boltzmann distribution and collision theory
Activation Energy, \( E_A \)
Activation energy is a key concept in collision theory and explains why not all particle collisions result in a chemical reaction.
The activation energy, \( E_A \), is the minimum energy required for a collision between particles to be effective and result in a chemical reaction.
![]()
Only particles that collide with energy equal to or greater than \( E_A \) can overcome the energy barrier and form products.
Role of Activation Energy
![]()
- Explains why some reactions occur slowly at low temperatures.
- Higher activation energy means fewer effective collisions at a given temperature.
- Lower activation energy means more particles have enough energy to react.
Activation Energy and Collision Theory
In terms of collision theory:
![]()
- Particles must collide.
- Collisions must have sufficient energy (≥ \( E_A \)).
- Collisions must have suitable orientation.
Example
Define activation energy.
▶️ Answer / Explanation
The activation energy is the minimum energy required for a collision to be effective.
Example
Explain why increasing temperature increases the rate of reaction in terms of activation energy.
▶️ Answer / Explanation
At higher temperature, more particles have energy equal to or greater than the activation energy, leading to more effective collisions.
Example
Explain why a reaction with a high activation energy proceeds slowly at room temperature.
▶️ Answer / Explanation
Only a small fraction of particles have enough energy to overcome the high activation energy, so few collisions are effective.
Boltzmann Distribution and Activation Energy
The Boltzmann distribution shows how the energies of particles in a system are distributed at a given temperature. It helps explain why only some particle collisions lead to a reaction.
The Boltzmann Distribution Curve

- The x-axis represents energy of particles.
- The y-axis represents number (or fraction) of particles with that energy.
- The curve starts at zero, rises to a peak, then tails off.
- The total area under the curve represents the total number of particles.
Activation Energy on the Boltzmann Distribution
The activation energy, \( E_A \), is shown on the energy axis as a vertical line.
![]()
- Particles with energy less than \( E_A \) cannot react.
- Particles with energy greater than or equal to \( E_A \) can take part in effective collisions.
- The area under the curve to the right of \( E_A \) represents the fraction of particles that can react.
Significance of Activation Energy
Activation energy explains why:
- Not all collisions result in a reaction.
- Reactions can be slow at low temperatures.
- Increasing temperature increases the rate of reaction.
Effect of Temperature
When temperature increases:
- The Boltzmann curve becomes broader and lower.
- The peak shifts to higher energy.
- The area beyond \( E_A \) increases significantly.
![]()
Result: a much larger fraction of particles have energy ≥ \( E_A \), so the rate of reaction increases.
Importantly, the value of \( E_A \) does not change with temperature.
Effect of Activation Energy Size
- A reaction with a large \( E_A \) has a very small area beyond \( E_A \).
- A reaction with a small \( E_A \) has a larger reacting fraction.
- This explains why reactions with high activation energy are slower.
How to Sketch a Boltzmann Distribution
- Draw axes and label energy (x-axis) and number of particles (y-axis).
- Sketch a curve starting at zero, rising to a peak, then tailing off.
- Draw a vertical line labelled \( E_A \).
- Shade the area to the right of \( E_A \) to show reacting particles.
Example
On a Boltzmann distribution, what does the area to the right of the activation energy represent?
▶️ Answer / Explanation
It represents the fraction of particles with enough energy to react.
Example
Explain why increasing temperature increases the rate of reaction using a Boltzmann distribution.
▶️ Answer / Explanation
Increasing temperature increases the area under the curve beyond \( E_A \), so more particles have enough energy for effective collisions.
Example
Two reactions occur at the same temperature but one has a higher activation energy. Use a Boltzmann distribution to explain which reaction is faster.
▶️ Answer / Explanation
The reaction with the lower activation energy has a larger fraction of particles beyond \( E_A \), so it has more effective collisions and a faster rate.
Effect of Temperature on the Rate of Reaction
An increase in temperature increases the rate of a chemical reaction. This can be explained using both the Boltzmann distribution and the frequency of effective collisions.
Explanation Using the Boltzmann Distribution
The Boltzmann distribution shows how particle energies are distributed at a given temperature.
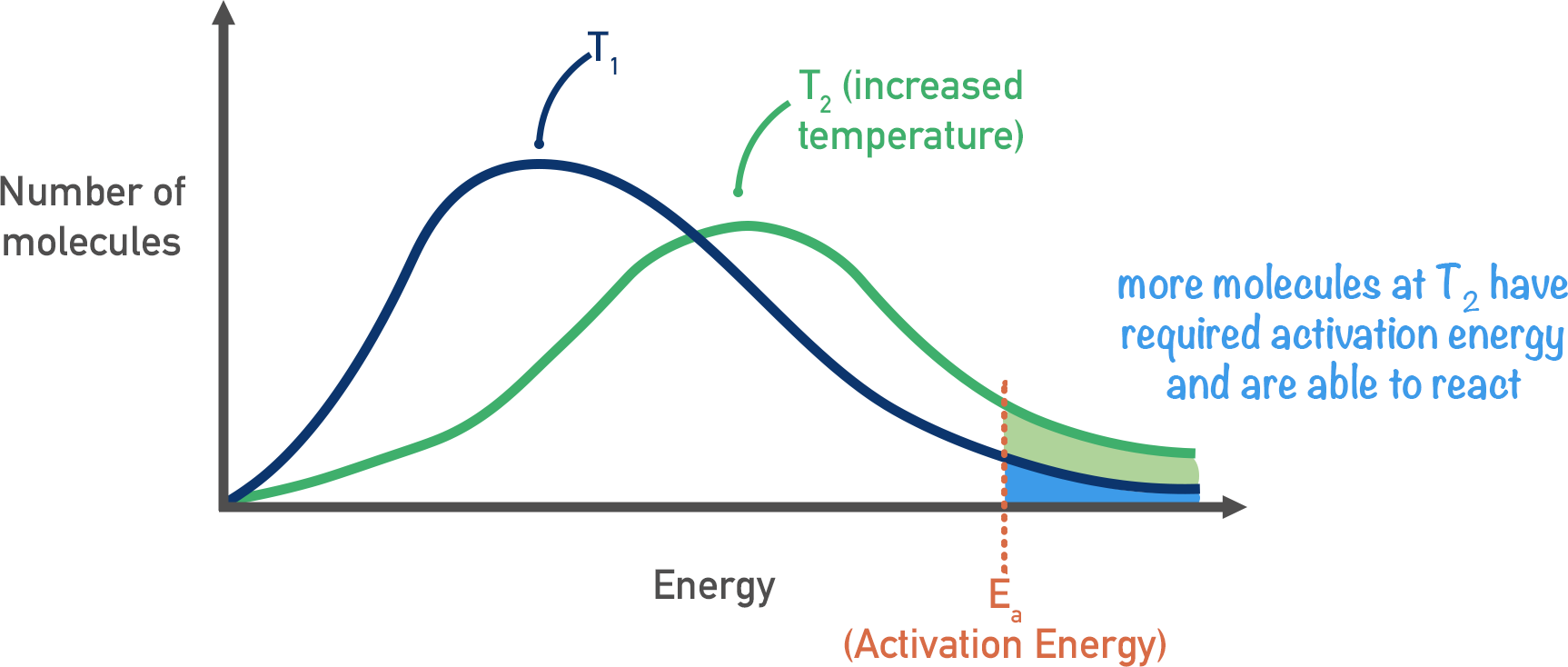
- At a higher temperature, the distribution curve becomes broader and lower.
- The peak shifts to higher energy.
- The total area under the curve remains constant.
The activation energy, \( E_A \), is shown as a vertical line on the energy axis.
- Particles with energy greater than or equal to \( E_A \) can react.
- Increasing temperature greatly increases the area under the curve beyond \( E_A \).
This means a much larger fraction of particles have sufficient energy to react.
Explanation Using Frequency of Effective Collisions
Increasing temperature increases the average kinetic energy of particles.![]()
- Particles move faster.
- Collisions occur more frequently.
- A higher proportion of collisions have energy ≥ \( E_A \).
Result: the frequency of effective collisions per unit time increases.
Why Rate Increases Rapidly with Temperature
Although collision frequency increases slightly with temperature, the main reason the rate rises sharply is:
- a large increase in the number of particles with energy ≥ \( E_A \)
- a large increase in the number of effective collisions
The value of the activation energy itself does not change with temperature.
Example
What happens to the area beyond the activation energy when temperature increases?
▶️ Answer / Explanation
The area increases, meaning more particles have enough energy to react.
Example
Explain why increasing temperature increases the number of effective collisions.
▶️ Answer / Explanation
At higher temperature, more particles have energy equal to or greater than the activation energy, so more collisions are effective.
Example
A small temperature increase causes a large increase in reaction rate. Explain this using both the Boltzmann distribution and collision theory.
▶️ Answer / Explanation
The Boltzmann distribution shows that a small temperature increase greatly increases the fraction of particles beyond \( E_A \).
This leads to a much higher frequency of effective collisions, causing a large increase in reaction rate.
