CIE AS/A Level Chemistry 8.3 Homogeneous and heterogeneous catalysts Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 8.3 Homogeneous and heterogeneous catalysts Study Notes – New Syllabus
CIE AS/A Level Chemistry 8.3 Homogeneous and heterogeneous catalysts Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
- explain and use the terms catalyst and catalysis:
(a) explain that a catalyst provides a different reaction pathway with lower activation energy
(b) explain this effect using the Boltzmann distribution
(c) construct and interpret reaction pathway diagrams with and without a catalyst
Catalysts and Catalysis
Catalysts increase the rate of chemical reactions without being used up. Their effect can be explained using activation energy, the Boltzmann distribution, and reaction pathway diagrams.
Definition of Catalyst and Catalysis

A catalyst is a substance that increases the rate of a chemical reaction without being consumed.
Catalysis is the process by which a catalyst increases the rate of a reaction.
- A catalyst is chemically unchanged at the end of the reaction.
- A catalyst does not affect the position of equilibrium.
(a) Effect of a Catalyst on Reaction Mechanism
In the presence of a catalyst:
![]()
- The reaction follows a different reaction mechanism.
- This mechanism has a lower activation energy, \( E_A \).
- The catalyst provides an alternative pathway.
This increases the number of effective collisions.
The enthalpy change of the reaction remains unchanged.
(b) Catalytic Effect Explained Using the Boltzmann Distribution
The Boltzmann distribution shows the spread of particle energies at a given temperature.
- The original reaction has a high activation energy \( E_A \).
- The catalysed reaction has a lower activation energy \( E_A^\text{cat} \).
On a Boltzmann distribution:
![]()
- The value of \( E_A \) moves left (to lower energy).
- The area under the curve beyond \( E_A \) increases.
- This means a greater fraction of particles can react.
Result: more effective collisions occur per second, increasing the rate.
(c) Reaction Pathway Diagrams (Energy Profile Diagrams)
A reaction pathway diagram shows how the potential energy of a system changes during a reaction.
Key features of a reaction pathway diagram:
![]()
- y-axis: potential energy
- x-axis: progress of reaction
- Peak represents the transition state
- Height of peak above reactants = activation energy
For a catalysed reaction:

- The catalysed pathway has a lower peak.
- The uncatalysed pathway has a higher peak.
- The energy difference between reactants and products is the same.
Comparison of Catalysed and Uncatalysed Reactions
| Feature | Uncatalysed | Catalysed |
|---|---|---|
| Activation energy | High | Lower |
| Reaction pathway | Single, high-energy route | Alternative lower-energy route |
| Rate of reaction | Slower | Faster |
Example
Define a catalyst.
▶️ Answer / Explanation
A catalyst is a substance that increases the rate of a reaction without being used up.
Example
Explain why a catalyst increases the rate of reaction using activation energy.
▶️ Answer / Explanation
The catalyst provides an alternative reaction pathway with a lower activation energy, so more collisions are effective.
Example
Use a Boltzmann distribution and a reaction pathway diagram to explain the effect of a catalyst.
▶️ Answer / Explanation
The catalyst lowers the activation energy shown on the energy profile diagram.
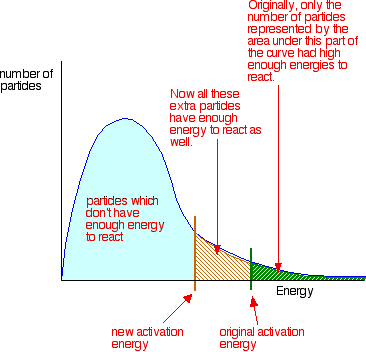
On the Boltzmann distribution, this increases the area beyond \( E_A \), meaning more particles can react.
