CIE AS/A Level Chemistry 9.1 Periodicity of physical properties of the elements in Period 3 Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 9.1 Periodicity of physical properties of the elements in Period 3 Study Notes – New Syllabus
CIE AS/A Level Chemistry 9.1 Periodicity of physical properties of the elements in Period 3 Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe trends in atomic radius, ionic radius, melting point and electrical conductivity
explain melting point and conductivity trends in terms of structure and bonding
Periodic Trends in Physical Properties of Elements
The physical properties of elements vary in a regular, repeating way across a period and down a group. These repeating patterns are called periodic trends and arise from changes in atomic structure.
Periodicity → repeating trends across periods and down groups
1. Atomic Radius
Atomic radius is half the distance between the nuclei of two bonded atoms of the same element.![]()
Variation across a period (left → right):
- Atomic radius decreases across a period.
- Number of shells remains constant.
- Nuclear charge increases → stronger attraction pulls electrons closer.
Variation down a group:
- Atomic radius increases down a group.
- Additional electron shells are added.
- Outer electrons are further from the nucleus.
2. Ionic Radius
Ionic radius is the radius of an ion in an ionic compound.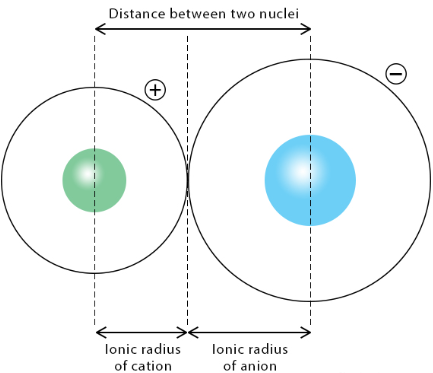
Cations (+ ions):
- Smaller than their parent atoms.
- Loss of electrons reduces electron–electron repulsion.
- Sometimes results in the loss of an entire shell.
Anions (− ions):
- Larger than their parent atoms.
- Extra electrons increase electron–electron repulsion.
- Electron cloud expands.
Trend: Ionic radius increases down a group due to additional shells.
3. Melting Point
Melting point depends on the strength of bonding or intermolecular forces between particles.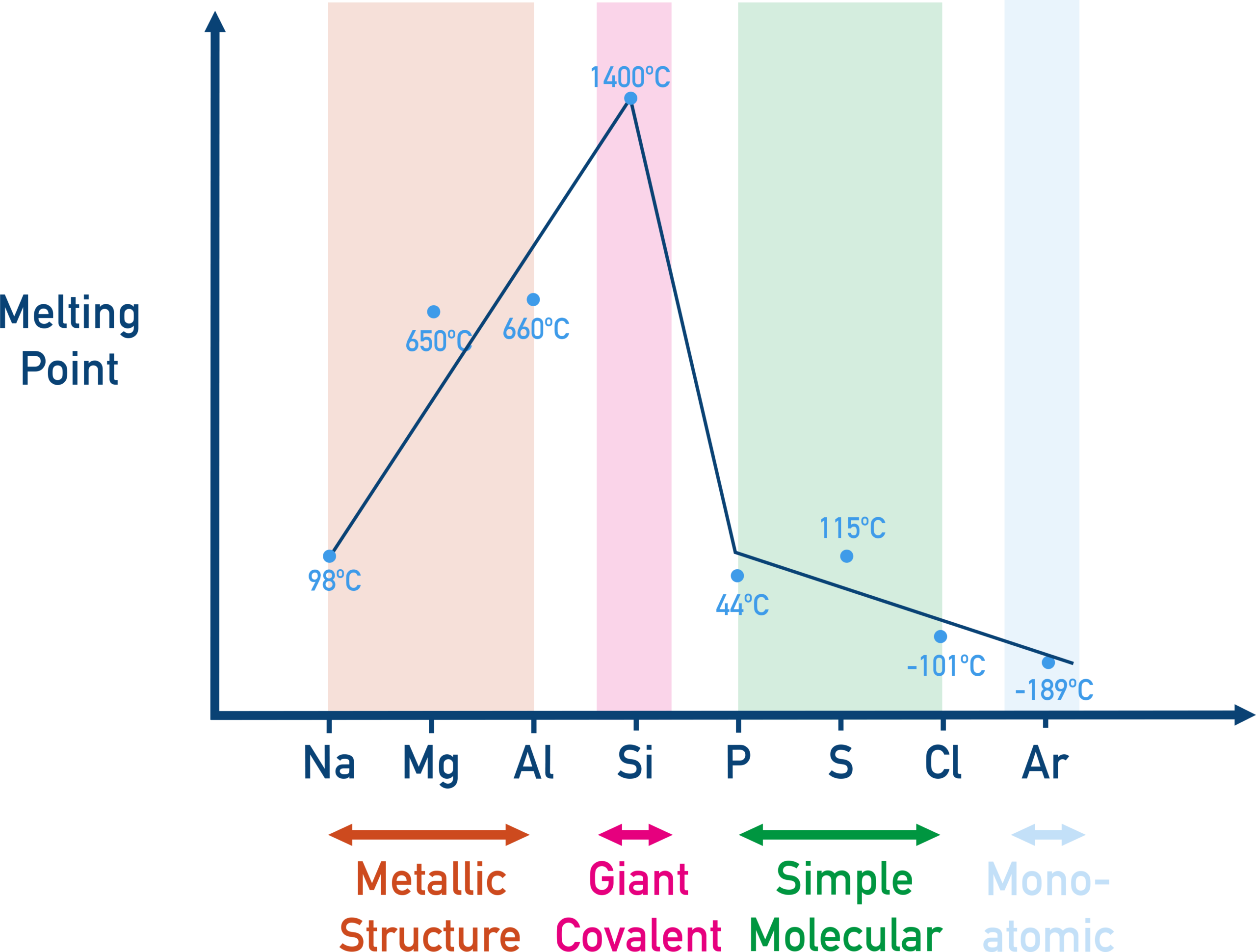
Across a period:
- Metals: melting point generally increases due to stronger metallic bonding.
- Giant covalent structures (e.g., carbon): very high melting point.
- Simple molecular substances: low melting points.
Down a group:
- Alkali metals: melting point decreases.
- Metallic bonding weakens as atomic radius increases.
4. Electrical Conductivity
Electrical conductivity depends on the presence of mobile charge carriers.
Across a period:
- Metals: good conductors due to delocalised electrons.
- Metalloids: intermediate conductivity.
- Non-metals: poor conductors or insulators.
Down a group (metals):
- Conductivity generally decreases.
- Atoms become larger and electron mobility decreases.
Summary Table
| Property | Across a Period | Down a Group |
|---|---|---|
| Atomic radius | Decreases | Increases |
| Ionic radius | Decreases (for same charge) | Increases |
| Melting point | Generally increases then decreases | Decreases (alkali metals) |
| Electrical conductivity | Decreases | Decreases (metals) |
Example
State how atomic radius changes across a period.
▶️ Answer / Explanation
Atomic radius decreases across a period because nuclear charge increases while the number of electron shells remains constant.
Example
Explain why a \( \mathrm{Na^+} \) ion is smaller than a sodium atom.
▶️ Answer / Explanation
\( \mathrm{Na^+} \) forms by losing one electron. This reduces electron–electron repulsion and removes the outer shell, causing the ion to be smaller than the atom.
Example (Hard)
Example
Describe and explain the trend in melting points across Period 3.
▶️ Answer / Explanation
Melting points increase from sodium to aluminium due to stronger metallic bonding. Silicon has a very high melting point because it has a giant covalent structure. Melting points then decrease sharply for phosphorus, sulfur, chlorine and argon because these are simple molecular substances with weak intermolecular forces.
Variation in Melting Point and Electrical Conductivity — Structure & Bonding
The melting point and electrical conductivity of elements depend on the type of structure present and the nature of bonding between particles. As structure and bonding change across periods and down groups, these physical properties also change in a predictable way.
Structure + bonding → strength of attractions → physical properties
1. Melting Point — Effect of Structure and Bonding
Melting point is the temperature at which particles gain enough energy to overcome the forces holding them together.
(a) Metals
- Metals have a giant metallic lattice.

- Positive metal ions are held together by strong attraction to delocalised electrons.
- More delocalised electrons and higher ionic charge → stronger metallic bonding.
- Stronger bonding → higher melting point.
(b) Giant covalent structures
- Atoms are linked by a large number of strong covalent bonds.
- A large amount of energy is needed to break these bonds.
- Results in very high melting points (e.g., carbon as diamond).
(c) Simple molecular substances
- Molecules are held together by weak intermolecular forces.
- Only weak forces need to be overcome on melting.
- Results in low melting points.
2. Electrical Conductivity — Effect of Structure and Bonding
Electrical conductivity requires the presence of mobile charge carriers.
(a) Metals
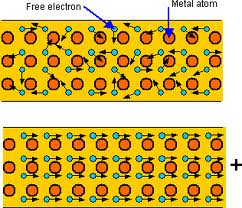
- Metals contain delocalised electrons that can move freely.
- These electrons carry charge through the lattice.
- Therefore, metals are good electrical conductors.
(b) Giant covalent structures
![]()
- Most have no mobile charged particles.
- Do not conduct electricity.
- Exception: graphite, which has delocalised electrons and can conduct.
(c) Simple molecular substances
- No free ions or delocalised electrons.
- Do not conduct electricity.
Summary Table
| Structure type | Bonding | Melting point | Electrical conductivity |
|---|---|---|---|
| Metallic lattice | Metallic bonding | High (variable) | Good |
| Giant covalent | Covalent bonding | Very high | Poor (except graphite) |
| Simple molecular | Intermolecular forces | Low | Poor |
Example
Why do metals generally have high melting points?
▶️ Answer / Explanation
Metals have a giant lattice held together by strong attraction between positive metal ions and delocalised electrons. A large amount of energy is required to overcome these forces, giving metals high melting points.
Example
Explain why silicon has a much higher melting point than phosphorus.
▶️ Answer / Explanation
Silicon has a giant covalent structure with many strong covalent bonds that must be broken on melting. Phosphorus exists as simple \( \mathrm{P_4} \) molecules with weak intermolecular forces, which require much less energy to overcome.
Example
Explain the variation in electrical conductivity across Period 3.
▶️ Answer / Explanation
Sodium, magnesium and aluminium are metals with delocalised electrons, so they conduct electricity well. Silicon is a giant covalent structure and has limited conductivity. Phosphorus, sulfur, chlorine and argon are simple molecular substances with no mobile charge carriers, so they do not conduct electricity.
