CIE AS/A Level Chemistry 9.2 Periodicity of chemical properties of the elements in Period 3 Study Notes- 2025-2027 Syllabus
CIE AS/A Level Chemistry 9.2 Periodicity of chemical properties of the elements in Period 3 Study Notes – New Syllabus
CIE AS/A Level Chemistry 9.2 Periodicity of chemical properties of the elements in Period 3 Study Notes at IITian Academy focus on specific topic and type of questions asked in actual exam. Study Notes focus on AS/A Level Chemistry latest syllabus with Candidates should be able to:
describe and write equations for reactions with:
oxygen → Na₂O, MgO, Al₂O₃, P₄O₁₀, SO₂
chlorine → NaCl, MgCl₂, AlCl₃, SiCl₄, PCl₅
water → Na and Mg onlystate and explain oxidation number trends in oxides and chlorides
describe reactions of oxides with water and predict pH
describe acid–base behaviour of oxides and hydroxides, including amphoteric behaviour of Al₂O₃ and Al(OH)₃
describe reactions of chlorides with water and predict pH
explain trends using bonding and electronegativity
suggest bonding types from observed properties
Reactions of Period 3 Elements with Oxygen, Chlorine and Water
Elements in Period 3 react with oxygen, chlorine and water in ways that reflect their metallic or non-metallic character. Moving from left to right across the period, reactions change from metallic to covalent in nature.
Metal → ionic compounds
Non-metal → covalent compounds
1. Reactions with Oxygen
Period 3 elements react with oxygen to form oxides.

- Metals form basic ionic oxides.
- Non-metals form acidic covalent oxides.
Equations:
\( \mathrm{4Na + O_2 \rightarrow 2Na_2O} \)
\( \mathrm{2Mg + O_2 \rightarrow 2MgO} \)
\( \mathrm{4Al + 3O_2 \rightarrow 2Al_2O_3} \)
\( \mathrm{P_4 + 5O_2 \rightarrow P_4O_{10}} \)
\( \mathrm{S + O_2 \rightarrow SO_2} \)
2. Reactions with Chlorine
Elements react with chlorine to form chlorides.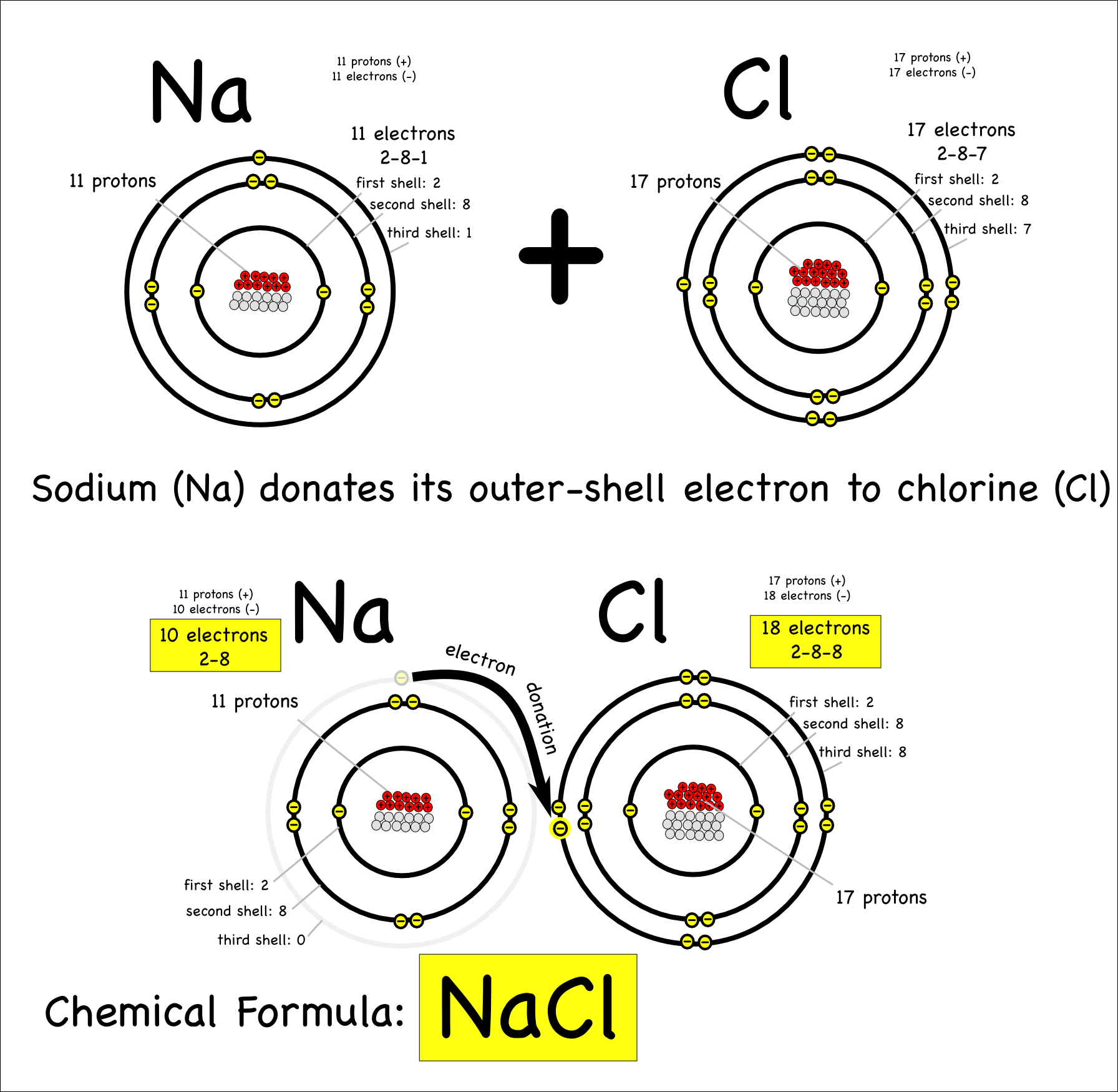
- Metals form ionic chlorides.
- Non-metals form covalent chlorides.
Equations:
\( \mathrm{2Na + Cl_2 \rightarrow 2NaCl} \)
\( \mathrm{Mg + Cl_2 \rightarrow MgCl_2} \)
\( \mathrm{2Al + 3Cl_2 \rightarrow 2AlCl_3} \)
\( \mathrm{Si + 2Cl_2 \rightarrow SiCl_4} \)
\( \mathrm{2P + 5Cl_2 \rightarrow 2PCl_5} \)
3. Reactions with Water (Sodium and Magnesium Only)

Sodium
- Reacts vigorously with cold water.
- Forms sodium hydroxide and hydrogen gas.
\( \mathrm{2Na + 2H_2O \rightarrow 2NaOH + H_2} \)
Magnesium
- Very slow reaction with cold water.
- Reacts more readily with steam.
Cold water: \( \mathrm{Mg + 2H_2O \rightarrow Mg(OH)_2 + H_2} \)
Steam: \( \mathrm{Mg + H_2O \rightarrow MgO + H_2} \)
Example
Write the equation for the reaction of magnesium with oxygen.
▶️ Answer / Explanation
\( \mathrm{2Mg + O_2 \rightarrow 2MgO} \)
Example
Explain why aluminium chloride is covalent whereas sodium chloride is ionic.
▶️ Answer / Explanation
Aluminium has a high charge density and polarises the chloride ion, leading to covalent bonding.
Sodium forms \( \mathrm{Na^+} \) ions easily and chloride forms \( \mathrm{Cl^-} \) ions, resulting in ionic bonding.
Example
Describe and compare the reactions of sodium and magnesium with water.
▶️ Answer / Explanation
Sodium reacts rapidly with cold water to form sodium hydroxide and hydrogen.
Magnesium reacts very slowly with cold water, forming magnesium hydroxide, but reacts more readily with steam to form magnesium oxide and hydrogen.
This difference is due to sodium being more reactive and having a lower ionisation energy than magnesium.
Variation in Oxidation Numbers of Period 3 Oxides and Chlorides
Across Period 3, the oxidation numbers of elements in their oxides and chlorides increase in a systematic way. This trend is explained by the increase in the number of electrons in the outer (valence) shell of the atoms.
Across a period → number of valence electrons increases → oxidation number increases
Key Principle
- Elements on the left of Period 3 tend to lose electrons.
- Elements on the right tend to share electrons.
- The maximum oxidation number corresponds to the number of outer-shell electrons.
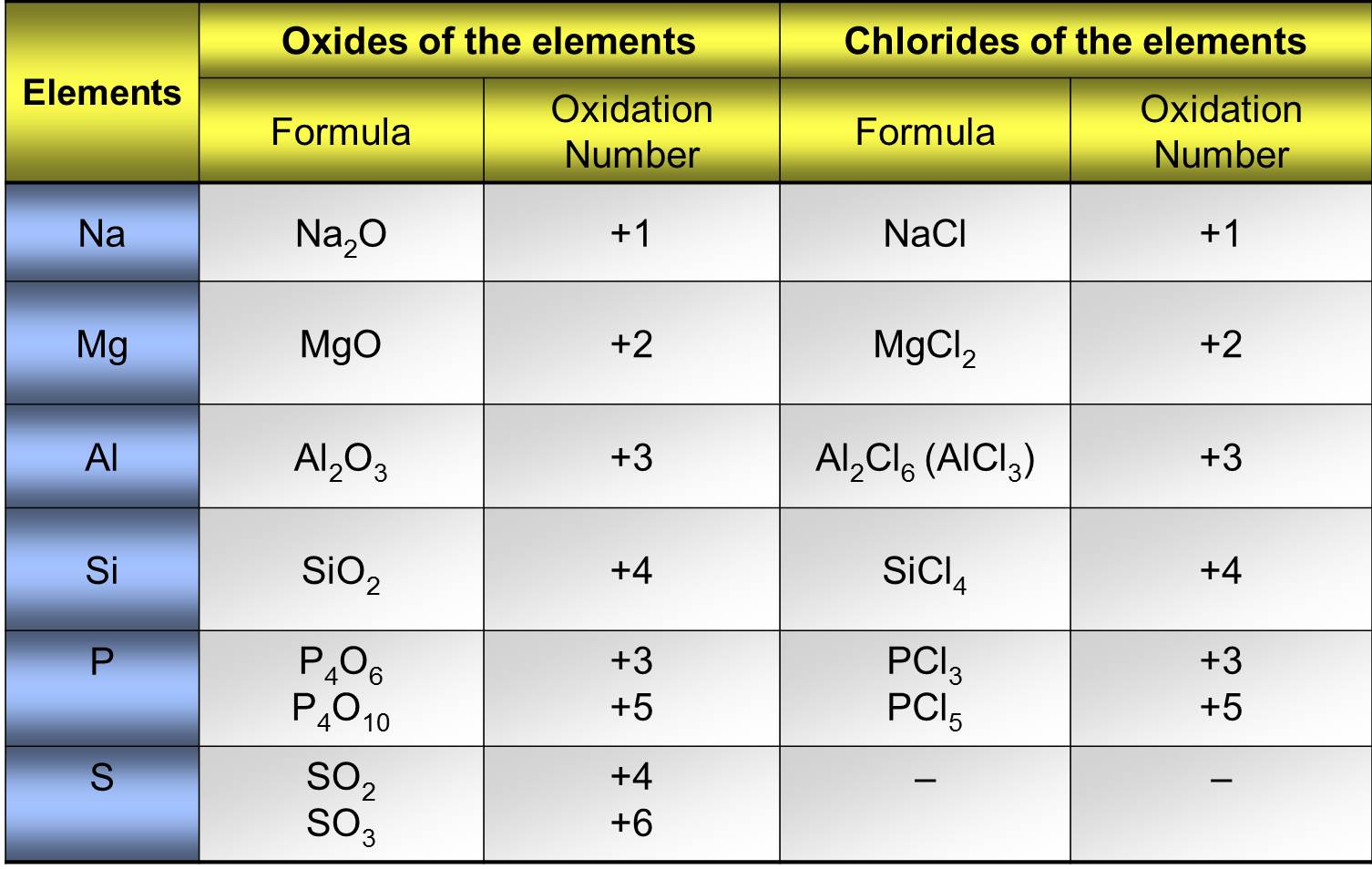
1. Oxides of Period 3 Elements
Oxygen has a fixed oxidation number of \( \mathrm{-2} \) in these oxides. The oxidation number of the Period 3 element therefore increases across the period.
\( \mathrm{Na_2O} \): Na = \( +1 \)
\( \mathrm{MgO} \): Mg = \( +2 \)
\( \mathrm{Al_2O_3} \): Al = \( +3 \)
\( \mathrm{P_4O_{10}} \): P = \( +5 \)
\( \mathrm{SO_2} \): S = \( +4 \)
\( \mathrm{SO_3} \): S = \( +6 \)
Explanation:
- Sodium has 1 valence electron → oxidation number \( +1 \).
- Magnesium has 2 valence electrons → oxidation number \( +2 \).
- Aluminium has 3 valence electrons → oxidation number \( +3 \).
- Phosphorus and sulfur use more outer-shell electrons in covalent bonding, giving higher oxidation numbers.
2. Chlorides of Period 3 Elements
Chlorine has a fixed oxidation number of \( \mathrm{-1} \). The oxidation number of the Period 3 element again increases across the period.
\( \mathrm{NaCl} \): Na = \( +1 \)
\( \mathrm{MgCl_2} \): Mg = \( +2 \)
\( \mathrm{AlCl_3} \): Al = \( +3 \)
\( \mathrm{SiCl_4} \): Si = \( +4 \)
\( \mathrm{PCl_5} \): P = \( +5 \)
Explanation:
- As the number of valence electrons increases, more electrons are involved in bonding with chlorine.
- This leads to progressively higher oxidation numbers.
- Metal chlorides are mainly ionic; non-metal chlorides are covalent.
Overall Trend
Across Period 3:
Valence electrons increase \( \rightarrow \) more electrons lost or shared
\( \rightarrow \) oxidation number increases
Example
State the oxidation number of magnesium in \( \mathrm{MgO} \).
▶️ Answer / Explanation
Oxygen is \( \mathrm{-2} \), so magnesium must be \( \mathrm{+2} \).
Example
Explain why aluminium has an oxidation number of \( +3 \) in both \( \mathrm{Al_2O_3} \) and \( \mathrm{AlCl_3} \).
▶️ Answer / Explanation
Aluminium has three electrons in its outer shell. It loses or shares all three electrons when forming compounds, giving it an oxidation number of \( +3 \).
Example
Explain why sulfur forms oxides with oxidation numbers \( +4 \) and \( +6 \), whereas sodium forms only \( +1 \) compounds.
▶️ Answer / Explanation
Sodium has only one outer-shell electron and can only lose one electron, giving an oxidation number of \( +1 \).
Sulfur has six valence electrons and can share different numbers of electrons in covalent bonding.
This allows sulfur to form compounds with oxidation numbers \( +4 \) (in \( \mathrm{SO_2} \)) and \( +6 \) (in \( \mathrm{SO_3} \)).
Reactions of Period 3 Oxides with Water
The oxides of Period 3 elements show a clear change from basic to acidic across the period. Their reactions with water, and the pH of the resulting solutions, depend on whether the oxide is ionic or covalent.
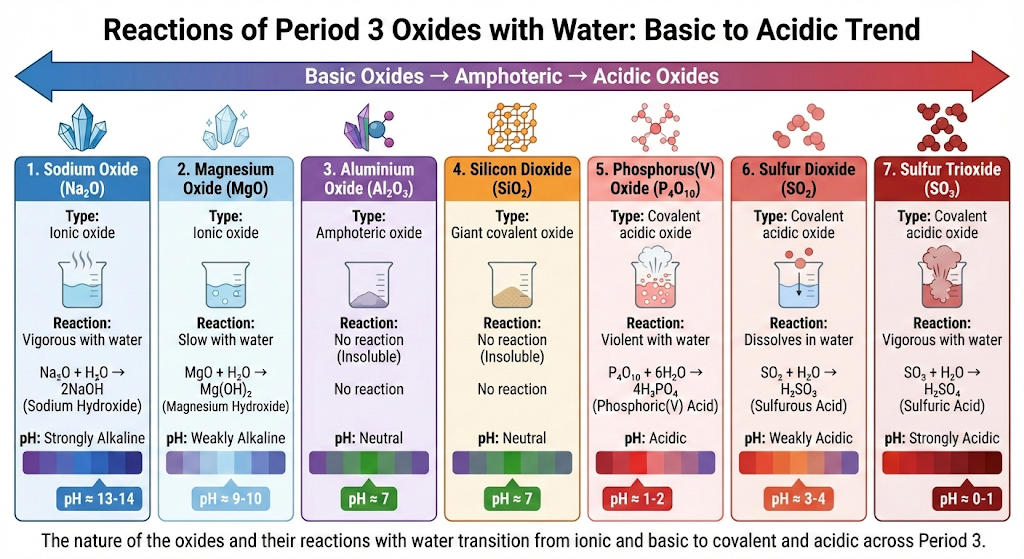
1. Sodium Oxide, \( \mathrm{Na_2O} \)
- Ionic oxide.
- Reacts vigorously with water.
- Forms a strongly alkaline solution.
\( \mathrm{Na_2O + H_2O \rightarrow 2NaOH} \)
Likely pH: \( \mathrm{pH \approx 13{-}14} \)
2. Magnesium Oxide, \( \mathrm{MgO} \)
- Ionic oxide.
- Reacts slowly with water.
- Forms a weakly alkaline solution.
\( \mathrm{MgO + H_2O \rightarrow Mg(OH)_2} \)
Likely pH: \( \mathrm{pH \approx 9{-}10} \)
3. Aluminium Oxide, \( \mathrm{Al_2O_3} \)
- Amphoteric oxide.
- Does not react with water.
- Insoluble and unreactive with water.
No reaction
Likely pH: neutral \( \mathrm{(pH \approx 7)} \)
4. Silicon Dioxide, \( \mathrm{SiO_2} \)
- Giant covalent oxide.
- Does not react with water.
- Insoluble due to strong covalent bonding.
No reaction
Likely pH: neutral \( \mathrm{(pH \approx 7)} \)
5. Phosphorus(V) Oxide, \( \mathrm{P_4O_{10}} \)
- Covalent acidic oxide.
- Reacts violently with water.
- Forms phosphoric(V) acid.
\( \mathrm{P_4O_{10} + 6H_2O \rightarrow 4H_3PO_4} \)
Likely pH: acidic \( \mathrm{pH \approx 1{-}2} \)
6. Sulfur Dioxide, \( \mathrm{SO_2} \)
- Covalent acidic oxide.
- Dissolves in water.
- Forms sulfurous acid.
\( \mathrm{SO_2 + H_2O \rightarrow H_2SO_3} \)
Likely pH: weakly acidic \( \mathrm{pH \approx 3{-}4} \)
7. Sulfur Trioxide, \( \mathrm{SO_3} \)
- Covalent acidic oxide.
- Reacts vigorously with water.
- Forms sulfuric acid.
\( \mathrm{SO_3 + H_2O \rightarrow H_2SO_4} \)
Likely pH: strongly acidic \( \mathrm{pH \approx 0{-}1} \)
Example
Write the equation for the reaction of sodium oxide with water and state the nature of the solution formed.
▶️ Answer / Explanation
\( \mathrm{Na_2O + H_2O \rightarrow 2NaOH} \)
The solution formed is strongly alkaline with \( \mathrm{pH \approx 13{-}14} \).
Example
Explain why \( \mathrm{Al_2O_3} \) does not react with water.
▶️ Answer / Explanation
\( \mathrm{Al_2O_3} \) has a very strong lattice with high lattice enthalpy and some covalent character.
This makes it insoluble and unreactive with water, so no reaction occurs.
Example
Describe the trend in pH of aqueous solutions formed by Period 3 oxides and explain the trend.
▶️ Answer / Explanation
Across Period 3, pH decreases from strongly alkaline to strongly acidic.
Metal oxides are ionic and form hydroxide ions in water, giving alkaline solutions.
Non-metal oxides are covalent and react with water to form acids, giving acidic solutions.
This reflects the change from metallic to non-metallic character across the period.
Acid–Base Behaviour of Period 3 Oxides and Hydroxides
Across Period 3, oxides and hydroxides change from basic to acidic. This change reflects the transition from metallic to non-metallic bonding and increasing covalent character.

1. Sodium Oxide, \( \mathrm{Na_2O} \)
- Strongly basic oxide.
- Contains oxide ions, \( \mathrm{O^{2-}} \).
- Reacts readily with acids.
Reaction with acid:
\( \mathrm{Na_2O + 2HCl \rightarrow 2NaCl + H_2O} \)
Explanation: Oxide ions act as bases by accepting protons.
2. Magnesium Oxide, \( \mathrm{MgO} \)
- Basic oxide.
- Less reactive than sodium oxide.
\( \mathrm{MgO + 2HCl \rightarrow MgCl_2 + H_2O} \)
Explanation: Ionic oxide neutralises acids to form salt and water.
3. Aluminium Oxide, \( \mathrm{Al_2O_3} \) (Amphoteric)
- Shows amphoteric behaviour.
- Reacts with both acids and bases.
With acids:
\( \mathrm{Al_2O_3 + 6HCl \rightarrow 2AlCl_3 + 3H_2O} \)
With sodium hydroxide:
\( \mathrm{Al_2O_3 + 2NaOH + 3H_2O \rightarrow 2Na[Al(OH)_4]} \)
Explanation: Aluminium oxide can both donate and accept hydroxide or oxide ions.
4. Phosphorus(V) Oxide, \( \mathrm{P_4O_{10}} \)
- Strongly acidic oxide.
- Acid anhydride of phosphoric(V) acid.
With base:
\( \mathrm{P_4O_{10} + 12NaOH \rightarrow 4Na_3PO_4 + 6H_2O} \)
5. Sulfur Dioxide, \( \mathrm{SO_2} \)
- Acidic oxide.
- Forms sulfite salts with bases.
\( \mathrm{SO_2 + 2NaOH \rightarrow Na_2SO_3 + H_2O} \)
6. Sulfur Trioxide, \( \mathrm{SO_3} \)
- Strongly acidic oxide.
- Acid anhydride of sulfuric acid.
\( \mathrm{SO_3 + 2NaOH \rightarrow Na_2SO_4 + H_2O} \)
Hydroxides
7. Sodium Hydroxide, \( \mathrm{NaOH} \)
- Strong alkali.
- Fully dissociates in water.
\( \mathrm{NaOH + HCl \rightarrow NaCl + H_2O} \)
8. Magnesium Hydroxide, \( \mathrm{Mg(OH)_2} \)
- Weak base due to low solubility.
\( \mathrm{Mg(OH)_2 + 2HCl \rightarrow MgCl_2 + 2H_2O} \)
9. Aluminium Hydroxide, \( \mathrm{Al(OH)_3} \) (Amphoteric)
With acids:
\( \mathrm{Al(OH)_3 + 3HCl \rightarrow AlCl_3 + 3H_2O} \)
With sodium hydroxide:
\( \mathrm{Al(OH)_3 + NaOH \rightarrow Na[Al(OH)_4]} \)
Explanation: Aluminium hydroxide reacts as both an acid and a base.
Example
State whether \( \mathrm{Na_2O} \) is acidic or basic and give a reason.
▶️ Answer / Explanation
\( \mathrm{Na_2O} \) is basic because it contains oxide ions that accept protons from acids.
Example
Write equations to show the amphoteric behaviour of aluminium oxide.
▶️ Answer / Explanation
With acid:
\( \mathrm{Al_2O_3 + 6HCl \rightarrow 2AlCl_3 + 3H_2O} \)
With base:
\( \mathrm{Al_2O_3 + 2NaOH + 3H_2O \rightarrow 2Na[Al(OH)_4]} \)
Example
Explain the trend in acid–base behaviour of Period 3 oxides from sodium to sulfur.
▶️ Answer / Explanation
Across Period 3, bonding changes from ionic to covalent.
Metal oxides contain oxide ions and are basic.
Aluminium oxide is amphoteric due to polarised bonding.
Non-metal oxides are covalent and acidic because they react with bases to form salts.
Reactions of Period 3 Chlorides with Water (Hydrolysis)
The chlorides of Period 3 elements show different behaviour with water depending on whether the bonding in the chloride is ionic or covalent. Across the period, chlorides change from ionic to covalent, leading to an increase in acidity of the solutions formed.

1. Sodium Chloride, \( \mathrm{NaCl} \)
- Ionic compound.
- Dissolves in water without reacting.
- Ions do not hydrolyse.
Dissolution only:
\( \mathrm{NaCl(s) \rightarrow Na^+(aq) + Cl^-(aq)} \)
Likely pH: neutral \( \mathrm{(pH \approx 7)} \)
Explanation: \( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) do not affect the pH of water.
2. Magnesium Chloride, \( \mathrm{MgCl_2} \)
- Mostly ionic.
- Slight hydrolysis of \( \mathrm{Mg^{2+}} \).
Dissolution:
\( \mathrm{MgCl_2(s) \rightarrow Mg^{2+}(aq) + 2Cl^-(aq)} \)
Hydration (partial):
\( \mathrm{[Mg(H_2O)_6]^{2+} \rightleftharpoons [Mg(H_2O)_5OH]^+ + H^+} \)
Likely pH: slightly acidic \( \mathrm{(pH \approx 6{-}6.5)} \)
Explanation: The small, doubly charged \( \mathrm{Mg^{2+}} \) ion polarises water slightly.
3. Aluminium Chloride, \( \mathrm{AlCl_3} \)
- Covalent with significant polarisation.
- Hydrolyses strongly in water.
Overall reaction:
\( \mathrm{AlCl_3 + 6H_2O \rightarrow [Al(H_2O)_6]^{3+} + 3Cl^-} \)
Hydrolysis:
\( \mathrm{[Al(H_2O)_6]^{3+} \rightleftharpoons [Al(H_2O)_5OH]^{2+} + H^+} \)
Likely pH: acidic \( \mathrm{(pH \approx 2{-}3)} \)
Explanation: The highly charged \( \mathrm{Al^{3+}} \) ion strongly polarises water, releasing \( \mathrm{H^+} \).
4. Silicon Tetrachloride, \( \mathrm{SiCl_4} \)
- Covalent molecular compound.
- Reacts vigorously with water (hydrolysis).
\( \mathrm{SiCl_4 + 2H_2O \rightarrow SiO_2(s) + 4HCl(aq)} \)
Likely pH: strongly acidic \( \mathrm{(pH \approx 1{-}2)} \)
Explanation: Hydrolysis produces hydrochloric acid.
5. Phosphorus Pentachloride, \( \mathrm{PCl_5} \)
- Covalent chloride.
- Hydrolyses violently with water.
Stepwise hydrolysis (overall):
\( \mathrm{PCl_5 + 4H_2O \rightarrow H_3PO_4 + 5HCl} \)
Likely pH: strongly acidic \( \mathrm{(pH \approx 0{-}1)} \)
Explanation: Hydrolysis produces both phosphoric acid and hydrochloric acid.
Example
State the pH of an aqueous solution of sodium chloride and give a reason.
▶️ Answer / Explanation
The pH is approximately \( \mathrm{7} \).
\( \mathrm{Na^+} \) and \( \mathrm{Cl^-} \) ions do not hydrolyse in water.
Example
Explain why aqueous \( \mathrm{MgCl_2} \) is slightly acidic.
▶️ Answer / Explanation
The \( \mathrm{Mg^{2+}} \) ion has a high charge density.
It polarises water molecules slightly, leading to the release of \( \mathrm{H^+} \) ions.
Example
Explain why \( \mathrm{AlCl_3} \) forms a more acidic solution in water than \( \mathrm{MgCl_2} \).
▶️ Answer / Explanation
\( \mathrm{Al^{3+}} \) has a higher charge and smaller radius than \( \mathrm{Mg^{2+}} \).
This gives \( \mathrm{Al^{3+}} \) a higher charge density, causing stronger polarisation of water molecules.
More \( \mathrm{H^+} \) ions are released, resulting in a lower pH.
Explaining Period 3 Trends Using Bonding and Electronegativity
The trends observed in Period 3 reactions and properties (Sections 9.2.2–9.2.5) can be explained by changes in bonding and electronegativity across the period.
Across Period 3:
Electronegativity increases → bonding changes from ionic to covalent
Electronegativity Trend Across Period 3

- Electronegativity increases from sodium to chlorine.
- Atoms become smaller with higher nuclear charge.
- Bonding changes from ionic (left) to covalent (right).
This single trend explains the behaviour of oxides, chlorides, melting points, conductivity and hydrolysis.
Melting Point and Electrical Conductivity
Bonding explanation:
- Metals (Na, Mg, Al) form giant metallic lattices.
- Strong metallic bonding → higher melting points.
- Delocalised electrons → good electrical conductivity.
As electronegativity increases:
- Bonding becomes covalent (Si onwards).
- Giant covalent structures (Si) have high melting points but poor conductivity.
- Simple molecular substances (P, S, Cl, Ar) have low melting points and no conductivity.
Reactions of Elements with Oxygen, Chlorine and Water
Oxides and chlorides:
- Low electronegativity metals form ionic compounds (e.g. \( \mathrm{Na_2O} \), \( \mathrm{NaCl} \)).
- Higher electronegativity elements form covalent compounds (e.g. \( \mathrm{SiCl_4} \), \( \mathrm{PCl_5} \)).
Water reactions:
- Ionic oxides react with water to form hydroxides (basic solutions).
- Covalent oxides react with water to form acids.
- This reflects increasing electronegativity and covalent character.
Acid–Base Behaviour of Oxides, Hydroxides and Chlorides
Bonding explanation:
- Ionic oxides contain \( \mathrm{O^{2-}} \) ions → strongly basic.
- As electronegativity increases, oxides become covalent and acidic.
- Aluminium compounds are amphoteric due to polarised bonding.
Chlorides:
- Ionic chlorides dissolve without reaction.
- Covalent chlorides hydrolyse in water to form acidic solutions.
- Higher electronegativity difference within molecules leads to hydrolysis.
Overall Explanation
- Increase in electronegativity
\( \Downarrow \) - Bonding becomes more covalent
\( \Downarrow \) - Oxides change from basic → amphoteric → acidic
- Chlorides change from ionic → hydrolysing covalent
Example
Why does electrical conductivity decrease across Period 3?
▶️ Answer / Explanation
Across Period 3, bonding changes from metallic to covalent.
Delocalised electrons are no longer present, so electrical conductivity decreases.
Example
Explain why the oxides become more acidic across Period 3.
▶️ Answer / Explanation
Electronegativity increases across Period 3.
Bonding becomes more covalent, and oxides react with water to form acids rather than bases.
Example
Explain why \( \mathrm{AlCl_3} \) hydrolyses in water but \( \mathrm{NaCl} \) does not.
▶️ Answer / Explanation
\( \mathrm{AlCl_3} \) has polar covalent bonding due to aluminium’s high charge density and electronegativity difference.
This causes hydrolysis and the release of \( \mathrm{H^+} \) ions.
\( \mathrm{NaCl} \) is ionic and its ions do not polarise water, so no hydrolysis occurs.
Deducing Bonding in Oxides and Chlorides from Physical and Chemical Properties
The type of bonding present in oxides and chlorides can be deduced from observations such as melting point, electrical conductivity, solubility and reactions with water. These properties reflect whether bonding is ionic, covalent, or giant covalent.
Observed properties → type of bonding
Key Bonding Types
- Ionic bonding: strong electrostatic attraction between oppositely charged ions.
- Covalent bonding: sharing of electron pairs between atoms.
- Giant covalent bonding: extended covalent network.
1. Using Melting Point
- Very high melting point → strong ionic lattice or giant covalent structure.
- Low melting point → simple molecular covalent substance.
Examples:
- \( \mathrm{Na_2O} \), \( \mathrm{MgO} \): very high melting points → ionic bonding.
- \( \mathrm{SiO_2} \): very high melting point → giant covalent.
- \( \mathrm{SO_2} \): low melting point → simple molecular covalent.
2. Using Electrical Conductivity
- Conducts when molten or in solution → ionic.
- Does not conduct in any state → covalent.
Examples:
- Molten \( \mathrm{NaCl} \) conducts → ionic bonding.
- \( \mathrm{SiCl_4} \) does not conduct → covalent bonding.
3. Using Solubility and Reactions with Water
- Dissolves without chemical reaction → ionic.
- Reacts with water (hydrolysis) → covalent.
\( \mathrm{NaCl(s) \rightarrow Na^+(aq) + Cl^-(aq)} \) (ionic)
\( \mathrm{SiCl_4 + 2H_2O \rightarrow SiO_2 + 4HCl} \) (covalent)
4. Using Acid–Base Behaviour
- Basic oxides → ionic with \( \mathrm{O^{2-}} \) ions.
- Acidic oxides → covalent.
- Amphoteric behaviour → polarised bonding.
Examples:
- \( \mathrm{Na_2O} \): basic → ionic.
- \( \mathrm{Al_2O_3} \): amphoteric → partial covalent character.
- \( \mathrm{SO_3} \): acidic → covalent.
Applying These Observations
By combining evidence from melting point, conductivity, solubility and chemical reactions, the bonding type can be confidently deduced.
Example
An oxide has a very high melting point and forms an alkaline solution in water. Suggest its bonding type.
▶️ Answer / Explanation
The high melting point and basic behaviour indicate an ionic oxide.
Example
A chloride dissolves in water without reacting and conducts electricity in solution. Deduce the bonding.
▶️ Answer / Explanation
The chloride dissociates into ions and conducts electricity.
This indicates ionic bonding.
Example
A colourless liquid chloride reacts violently with water to form acidic fumes and does not conduct electricity. Suggest the bonding and justify your answer.
▶️ Answer / Explanation
The substance is a liquid, does not conduct electricity, and reacts with water.
These properties indicate a simple molecular covalent chloride, such as \( \mathrm{SiCl_4} \) or \( \mathrm{PCl_5} \).
