Question
Different polysaccharides are used by plants for energy storage and structural support. The molecular structures for two common polysaccharides are shown in Figure 1. Starch is used by plants for energy storage, and cellulose provides structural support for cell walls. The monomer used to construct both molecules is glucose.

A study determined the effect of two different digestive enzymes, A and B, on these two polysaccharides. Table 1 presents the data from the study.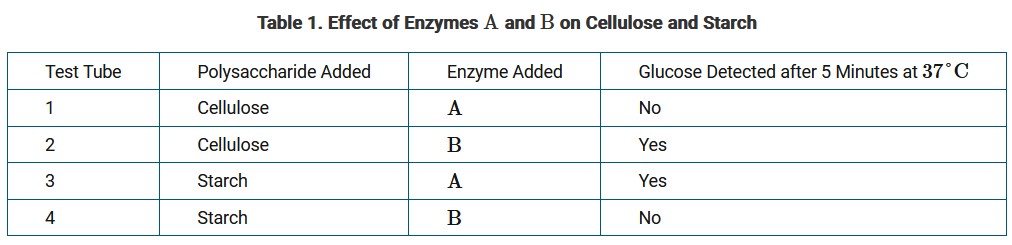
Mammals do not produce digestive enzyme B. However, sheep and cattle are two types of mammals that contain microorganisms in their digestive tract that produce enzyme B.
Which of the following best describes the process that adds a monosaccharide to an existing polysaccharide?
A. The monosaccharide is completely broken down by a specific enzyme and then the atoms are reorganized and made into a polysaccharide.
B. Ionic bonds are formed between adjacent carbon atoms of the monosaccharide and the polysaccharide by adding water (\(H_2O\)) and a specific enzyme.
C. A specific enzyme removes the hydrogen (H) from the monosaccharide and the hydroxide (OH) from the polysaccharide, creating a bond between the two and creating a water (\(H_2O\)) molecule.
D. A specific enzyme removes two hydroxides (OH), one from the monosaccharide, and one from the polysaccharide, creating a bond between the two monosaccharides and creating a hydrogen peroxide (\(H_2O_2\)) molecule.
▶️Answer/Explanation
Ans: C
This is a description of dehydration synthesis, which joins multiple monosaccharides to create a polysaccharide and produces water (\(H_2O\)) molecules.
Question
Which of the following best describes the differences between saturated and unsaturated lipids?
(A) Saturated lipids have at least one C=C double bond and tend to be
solid at room temperature. Unsaturated lipids have no double
bonds and tend to be liquid at room temperature.
(B) Saturated lipids have at least one C=C double bond, which makes
them amphipathic. Unsaturated lipids have no double bonds and
are hydrophilic.
(C) Saturated lipids have no C=C double bonds and tend to be solid at
room temperature. Unsaturated lipids have at least one C=C
double bond and tend to be liquid at room temperature.
(D) Saturated and unsaturated lipids both have C=C double bonds.
Saturated lipids are hydrophobic, and unsaturated lipids are
hydrophilic.
▶️Answer/Explanation
Ans:
(C) Saturated lipids have carbon atoms linked through single bonds
and form straight chains; this makes saturated lipids easier to pack
tightly and more likely to be solid at room temperature. Choice (A) is
incorrect because saturated lipids do not have C=C double bonds.
Choice (B) is incorrect because saturated lipids are not amphipathic
(phospholipids are amphipathic). Choice (D) is incorrect because while
unsaturated lipids do have C=C double bonds, saturated lipids do not.
Also, both saturated and unsaturated lipids are hydrophobic.
Question
The molecular formula for glucose is \(C_6 H_{12} O_6\). The molecule maltose is formed by a dehydration reaction that links two glucose molecules together. What is the molecular formula for maltose?
(A) \(C_2 H_4 O_2\)
(B) \(C_6 H_{10} O_5\)
(C) \(C_{12} H_{22} O_{11}\)
(D) \(C_{12} H_{24} O_{12}\)
▶️Answer/Explanation
Ans:
(C) A dehydration reaction removes a water molecule. Two glucose
molecules would contain 12 carbon atoms, 24 hydrogen atoms, and 12
oxygen atoms, but the dehydration reaction (linking the two glucose molecules to form maltose) would remove two hydrogen atoms and
one oxygen atom to form the water molecule removed in a dehydration
reaction. This leaves 12 carbon atoms but only 22 hydrogen atoms and
11 oxygen atoms. Choice (A) is incorrect because it has fewer atoms
than a single glucose molecule, so it could not be maltose (which is
made from two glucose molecules). Choice (B) is incorrect because it
is what would remain if a water molecule was removed from a single
glucose molecule, not from two glucose molecules. Choice (D) is
incorrect because it shows the number of atoms present in two glucose
molecules without taking into account the atoms lost from the water
molecule that was removed in the dehydration synthesis reaction.
Question
A nutrient is considered essential if:
(A) the body requires it to function properly.
(B) all cells require it to function properly.
(C) the body can synthesize it only in small amounts.
(D) it must be obtained in the diet.
▶️Answer/Explanation
Ans: D
An essential nutrient is one that must be obtained in the diet. Many
molecules are required for cells (or the body) to function, but if it can be
made by the organisms that requires it, it is not considered an “essential
nutrient.”
Question
The half-life of a substance is the time it takes for one-half of the number of molecules of the substance to be removed, degraded, or modified. All
proteins and most molecules in the body have a specific half-life. The following graphs show the relative concentrations of intracellular
molecules after the synthesis of that molecule has increased or decreased 10-fold according to its specific half-life. (The data were adapted from
Alberts, Johnson, Lewis, Raff, Roberts, and Walter, Molecular Biology of the Cell, 4th edition, 2002.)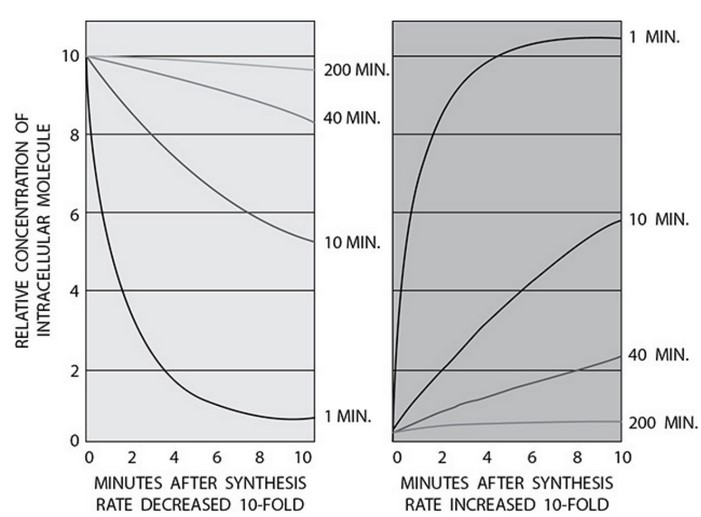
Which of the following half-lives would be expected for a regulatory molecule and why?
(A) short half-life because its concentration rapidly changes with
synthesis and rapidly disappears when synthesis is slowed or
stopped
(B) long half-life because its concentrations remain relatively stable
regardless of synthesis rates
(C) moderate half-life because its concentrations remain relatively
stable but can change when needed
(D) moderate half-life because the maximum amount of the
molecule will be present regardless of the synthesis rate
▶️Answer/Explanation
Ans: A
A short half-life is a key characteristic of a regulatory molecule.
The protein with the shortest half-life in the graphs has a half-life of 1
minute. When production decreases ten-fold, the relative concentration
(relative to its initial concentration in the cell) drops to nearly its lowest
levels within 5 minutes and drops to its lowest level by 8–10 minutes. The
rapid decrease in concentration allows a quick response from the molecule
that responds to that molecule. When synthesis rates increase ten-fold, the
concentration rises to near maximum levels in 4–5 minutes, allowing for a
quick response. If the half-life of the molecule were long, the process would
take longer to activate and then be activated for a longer time.
