Question
Refer to the process of sodium and potassium transport.
See the following equation.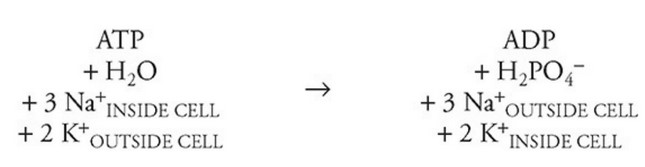
The average cell expends from 20%–40% of energy on maintaining concentration differences of sodium and potassium ions across cell
membranes. Intracellular potassium ion \((K^+)\) concentrations are maintained at approximately 140 mM. Extracellular sodium ion \((Na^+)\) concentrations are maintained between 5 and 15 mM.
(A) Identify a cell type that you think expends a greater fraction (than 20%–40%) of its energy on sodium/potassium transport.
(B) Explain why the energy requirement to maintain \(K^+/Na^+\) concentrations are higher in this cell type and describe the conditions under which the greatest fraction of energy expenditure on \(Na^+/K^+\) transport is expected.
▶️Answer/Explanation
Ans:
Muscle (including cardiac) cells and neurons are electrically
excitable cells. They use the Na+ and K+ ion gradients to depolarize their
membranes. Active neurons and muscle tissue are expected to have the
highest rate of energy expenditure because they are continually “releasing”
their gradients through opening channels and then pumping the ions back to
the side of the membrane in which they are more concentrated.
Neurons that are firing many action potentials and muscle cells that have
a high frequency of contraction are expected to use an even greater fraction
of their energy on maintaining Na+/K+ gradients. Up to 70% of the energy
expenditure in neurons is spent on maintaining Na+/K+gradients.
Question
Refer to ouabain, an inhibitor of the \(Na^+, K^+\)-ATPase transporter, and the following diagram and table.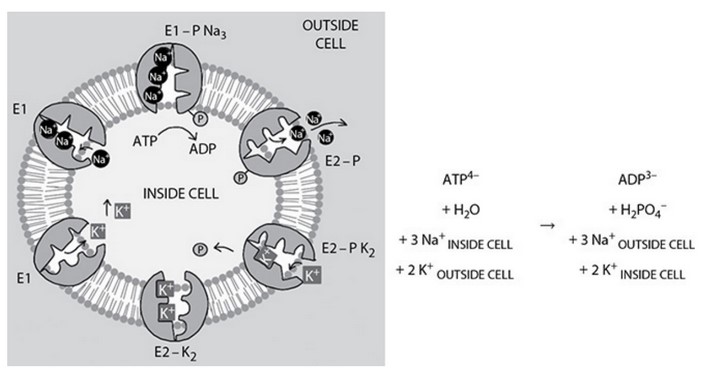
Certain plant and animal steroids such as ouabain inhibit the \(Na^+, K^+\)-ATPase ion transporter by binding to the transporter when it is in the \(E_2-P\) state, increasing its stability in this state.
(A) State the effect this could have on the transport of \(Na^+\) and \(K^+\). Justify your answer.
(B) Propose a potentially useful application of this molecule and briefly describe how or why it could be beneficial.
▶️Answer/Explanation
Ans:
The stabilization of the \(Na^+, K^+\)-ATPase by ouabain inhibits transport.
The increased stability of the pump makes it move to the next position
because it has a lower energy. Transitioning to the next configuration would require a greater input of energy to occur (see a picture illustrating the relationship between energy and stability after answer 125).
Ouabain would be very useful in in vitro studies of ion transport or the \(Na^+, K^+\)-ATPase transporter. It would be helpful in studying cells, like muscle and neurons, that use \(Na^+\) and \(K^+\) flux across the membrane to function. It may be useful in vivo to temporarily stop a particular muscle
cell or neuron from functioning and then examine the effects. Finally, the study of ouabain would be helpful in finding an antidote to ouabain and ouabain-like compounds.
Question
Refer to ouabain, an inhibitor of the \(Na^+, K^+\)-ATPase transporter, and the following diagram and table.
In people with hypertension (high blood pressure) there is an accumulation of sodium (and calcium) in the cells that line the blood vessels that results in the narrowing of the blood vessels. The accumulation of intracellular sodium ion concentrations is directly caused by an inhibition of the \(Na^+, K^+\)-ATPase transporter in the cells that line the blood vessels.
The chemical responsible for the inhibition of the \(Na^+, K^+\) -ATPase transporter is present at very low concentrations in the plasma of people with hypertension. Mass spectrometric analysis has revealed the molecule is very close or identical to ouabain.
The structures of three cardiac glycosides (also called cardiotonic steroids)—ouabain, strophanthidin, and digitoxigenin—are shown at the top
of the next page. These molecules all bind to the \(Na^+, K^+\)-ATPase transporter.
(A) Indicate the part of the ouabain molecule that is most likely the part that acts on the \(Na^+, K^+\)-ATPase.
(B) Briefly state your reasoning.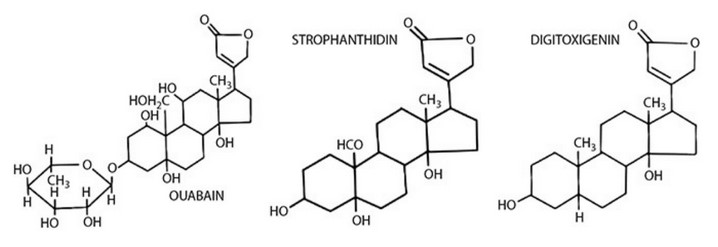
▶️Answer/Explanation
Ans:
All three molecules share the same basic structure—the steroid nucleus and the 4-carbon oxygen-containing ring, so that is likely the part of the molecule that binds to the \(Na^+\), K-ATPase transporter.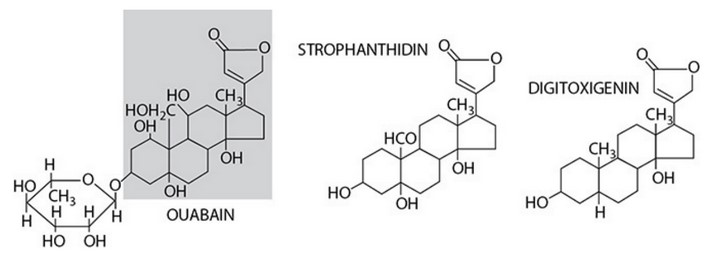
The sugar attached to ouabain does not affect its binding properties but
does increase its water-solubility, making it more soluble in the plasma.
