Question
Researchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH. The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C
. The results of the experiment are represented in Figure 1.

▶️Answer/Explanation
Ans: D
A negative control is a group in which no response is expected. It is the opposite of the positive control, in which a known response is expected. Heat-denatured peroxidase will be inactive. Repeating the experiment using heat-denatured peroxidase will allow the researcher to measure reaction rates in the presence of inactive peroxidase.
Question
esearchers investigated the influence of environmental pH on the activity of peroxidase, an enzyme that catalyzes the conversion of hydrogen peroxide to water and oxygen gas. In an experiment, the researchers added a hydrogen peroxide solution containing guaiacol to several identical test tubes and adjusted the solution in each test tube to a different pH. The researchers included the guaiacol because it caused the solutions to change color as the reactions proceeded, which the researchers relied on for measuring reaction rates. Finally, the researchers added the same amount of peroxidase to each test tube and measured the rate of each reaction at 23°C
. The results of the experiment are represented in Figure 1.

▶️Answer/Explanation
Ans: C
In the original experiment, the researchers used guaiacol as a reaction indicator. By using oxygen gas production to measure reaction rates, the researchers no longer need the guaiacol and can repeat the experiment without it, which will reveal the effect of guaiacol on the reaction rates.
Question
Catalase is an enzyme found in yeast cells that facilitates the chemical breakdown of hydrogen peroxide to water and oxygen gas. An experiment was conducted to determine the effect of pH on catalase function. Five buffer solutions of varying pH (2, 4, 6, 8, and 10) were prepared and added to separate test tubes. Hydrogen peroxide (H2O2)
was added to each test tube. Yeast was added, and the reactions were timed. After 1 minute the amount of oxygen gas released was determined by measuring the foam layer produced in each test tube. Figure 1 illustrates the experimental setup.

▶️Answer/Explanation
Ans: A
Since the experiment is studying the effect of pH on catalase in yeast, the control set should determine what would happen without yeast present. Each test tube in the control set should contain hydrogen peroxide and one of the pH solutions.
Question
A student designs an experiment to investigate the influence of temperature on enzyme function. The student’s plan is presented in Table 1.
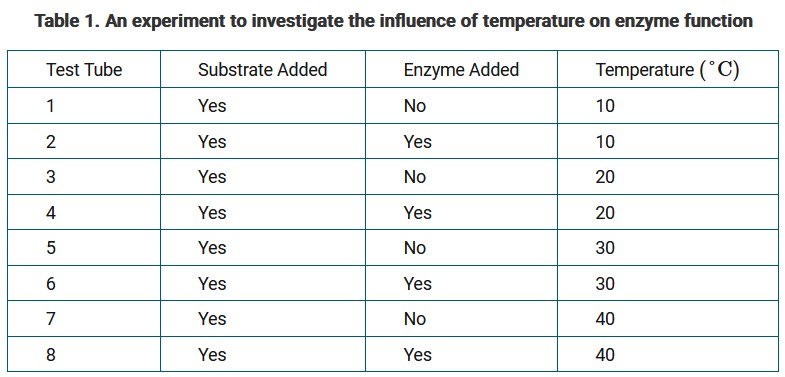
Which test tubes are controls in the experiment?
A. Test tubes 1 and 2 only
B. Test tubes 5 and 6 only
C. Test tubes 1, 3, 5, and 7
D. Test tubes 2, 4, 6, and 8
▶️Answer/Explanation
Ans: C
The test tubes that do not contain the enzyme are negative controls in the experiment. The negative controls help confirm that the results of the experiment are related to enzyme function and not some other factor.
Question
A researcher designs an experiment to investigate whether soil bacteria trigger the synthesis of defense enzymes in plant roots. The design of the experiment is presented in Table 1. For each group in the experiment, the researcher will determine the average rate of change in the amount of defense enzymes in the roots of the seedlings.
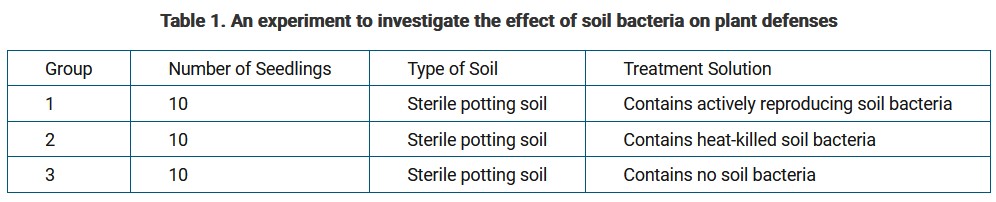
Which of the following statements best helps justify the inclusion of group 2 as one of the controls in the experiment?
A. It will show whether the changes observed in group 1 depend on the metabolic activity of soil bacteria.
B. It will show whether the changes observed in group 1 depend on the type of plants used in the experiment.
C. It will show the average growth rate of seedlings that are maintained in a nonsterile environment.
D. It will show the changes that occur in the roots of seedlings following an infection by soil bacteria.
▶️Answer/Explanation
Ans: A
The seedlings in group 1 will be treated with a solution that contains actively reproducing bacteria, which will be metabolically active. In contrast, the seedlings in group 2 will be treated with a solution that contains heat-killed bacteria, which will be metabolically inactive. The researcher will be able to compare the results for group 1 with those for group 2. The comparison will help the researcher determine whether the changes observed in group 1 depend on the metabolic activity of soil bacteria.
