Question
Enzymes are important biological molecules.
(a) Describe how an enzyme interacts with a substrate.
(b) Explain the difference between competitive inhibitors and allosteric inhibitors of enzymes.
(c) An enzyme has its maximum efficiency at an optimum temperature of 25° Celsius. Predict what effect a decrease in temperature to 5° Celsius would have on the enzyme’s efficiency.
(d) Justify your prediction from part (c).
▶️Answer/Explanation
Ans:
(a) Enzymes interact with a specific substrate that has properties (such
as shape and charge) that are compatible with those at the enzyme’s
active site.
(b) Competitive inhibitors bind to enzymes at the active site, whereas
allosteric inhibitors bind to enzymes at the allosteric site.
(c) A decrease in temperature to 5° Celsius would decrease the
enzyme’s efficiency.
(d) Decreases in temperature reduce the number of molecular
collisions between the substrate and the enzyme, reducing the
number of chemical reactions and the enzyme’s efficiency.
Decreases in temperature may also alter the tertiary structure of the
enzyme, altering its active site and reducing its catalytic ability.
Question
The enzyme catalase breaks down hydrogen peroxide into water and oxygen, as shown in this equation:
\(2H_2O_{2(l)} \Rightarrow 2H_2O_{(l)} + O_{2(g)}\)
An experiment was performed to measure the amount of oxygen bubbles produced at two different temperatures. The data are shown in
the table.
| Time (minutes) | Milliliters of Oxygen Produced at \(37^o\) Celsius | Milliliters of Oxygen Produced at \(45^o\) Celsius |
| 0 | 0 | 0 |
| 5 | 2.5 | 0.5 |
| 10 | 5.2 | 0.8 |
| 15 | 7.7 | 1.0 |
| 20 | 10.3 | 1.1 |
(a) Calculate the rate of the enzyme-catalyzed reaction at both temperatures for the final 10 minutes of the experiment.
(b) Predict which temperature (37°C or 45°C) is closer to the optimum temperature for this enzyme.
(c) Justify your prediction from part (b) using the data provided.
(d) In a follow-up experiment, the reaction was performed at a temperature of 50°C and no oxygen was produced. Explain why
the enzyme would not function at 50°C.
▶️Answer/Explanation
Ans:
(a) At 37°C, the rate of the enzyme-catalyzed reaction for the final 10 minutes of the experiment was
\(\frac{(10.3-5.2)milliliters}{10 minutes}=0.51 \frac{milliliters}{minute}\). At 45°C, the rate of the
enzyme-catalyzed reaction for the final 10 minutes of the
experiment was \(\frac{(1.1-0.8}milliliters}{10 minutes}=0.03 \frac{milliliters}{minute}\)
(b) The optimum temperature for this enzyme is closer to 37°C.
(c) The rate of the reaction is greater at 37°C than at 45°C, so 37°C is
closer to the optimum temperature for this enzyme.
(d) At 50°C, the enzyme was probably denatured. High temperatures
can denature enzymes, changing their shape so that they no longer
function.
Question
Amylase is an enzyme that catalyzes the breakdown of amylose into its glucose subunits. The activity of amylase was measured at 37° Celsius
and a pH of 7, both of which are optimum conditions for the activity of this enzyme. The production of glucose was measured both with and
without the presence of compound X. Data are shown in the following table:
| Time (min) | Cumulative Amount of Glucose Produced (millimoles) in the Absence of Compound X | Cumulative Amount of Glucose Produced (millimoles) is the Presence of Compound X |
| 0 | 0 | 0 |
| 5 | 5.6 | 7.5 |
| 10 | 12.0 | 16.7 |
| 15 | 17.0 | 24.0 |
| 20 | 22.1 | 31.4 |
(a) On the axes provided, construct an appropriately labeled graph of this data.
(b) Based on the data and your graph from part (a), identify compound X as either: a cofactor, a competitive inhibitor, or a
noncompetitive inhibitor of amylase. Justify your answer with evidence from the data.
(c) Construct an additional line on your graph from part (a) that represents your prediction as to the expected experimental results
at a temperature of 10° Celsius in the absence of compound X.
(d) Explain your prediction from part (c), stating why you placed the additional line where you did.
▶️Answer/Explanation
Ans:
(a) 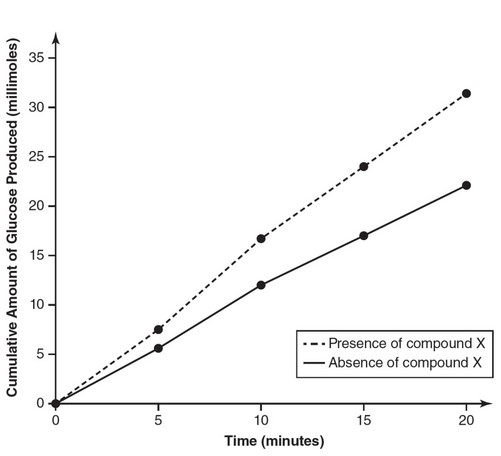
(b) Compound X is likely a cofactor. Cofactors increase enzyme
efficiency. Because the amount of product produced in the presence
of compound X is greater than the amount of product produced in
the absence of compound X, compound X is most likely a cofactor
of amylase.
(c) 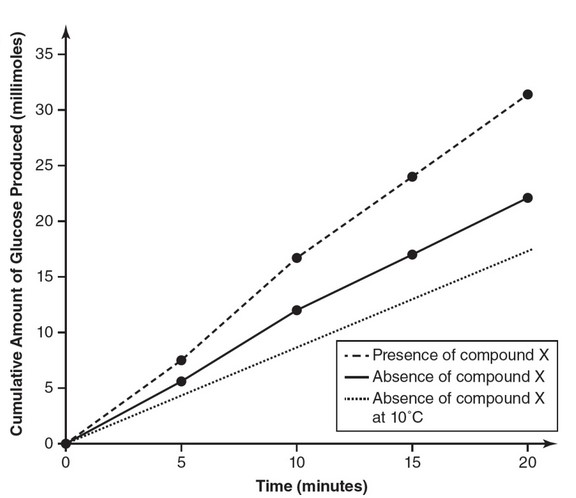
(d) At reduced temperatures, there are fewer molecular collisions
between the enzyme and the substrate as a result of the reduced
kinetic energy at lower temperatures. Therefore, it is reasonable to
predict that the reaction rate at 10°C would be slower than that of
either 37°C measurement and thus that line is lower on the graph
than the other two lines.
