AP Biology 1.7 Proteins Study Notes - New Syllabus Effective 2025
AP Biology 1.7 Proteins Study Notes- New syllabus
AP Biology 1.7 Proteins Study Notes – AP Biology – per latest AP Biology Syllabus.
LEARNING OBJECTIVE
Describe the structure and function of proteins.
Key Concepts:
- Proteins
1.7.A.1 – Structure of Proteins
Proteins are polymers made of amino acids linked by peptide bonds.
🧱 Monomers: Amino Acids
- Amino group (–NH₂)
- Carboxyl group (–COOH)
- Central carbon (α-carbon)
- R-group (side chain) → gives each amino acid its unique properties
🔗 Amino acids are joined by dehydration synthesis, forming peptide bonds.
🧗♂️ Levels of Protein Structure
| Level | Description | Example |
|---|---|---|
| Primary | Linear sequence of amino acids (polypeptide chain) | Like spelling letters in a word |
| Secondary | Local folding into α-helices or β-sheets due to hydrogen bonds | Spring-like coil or pleated sheet |
| Tertiary | 3D shape from R-group interactions (H-bonds, ionic, hydrophobic) | Fully folded protein |
| Quaternary | 2+ polypeptide chains bonded together | Hemoglobin (4 subunits) |
🔧 Functions of Proteins
Proteins are extremely diverse in function:
- Structure (e.g., collagen, keratin)
- Transport (e.g., hemoglobin carries oxygen)
- Enzymes (speed up reactions — e.g., lactase, amylase)
- Defense (e.g., antibodies)
- Signaling (e.g., insulin hormone)
- Movement (e.g., actin & myosin in muscles)
🧠 Summary:
| Component | Key Point |
|---|---|
| Monomer | Amino acids |
| Bond | Peptide bond |
| Built by | Dehydration synthesis |
| Function | Enzymes, structure, transport, etc. |
| Structure Levels | Primary → Quaternary |
1.7.A.1 – Protein Structure: Peptide Chains
🧱 Proteins Are Built from Amino Acids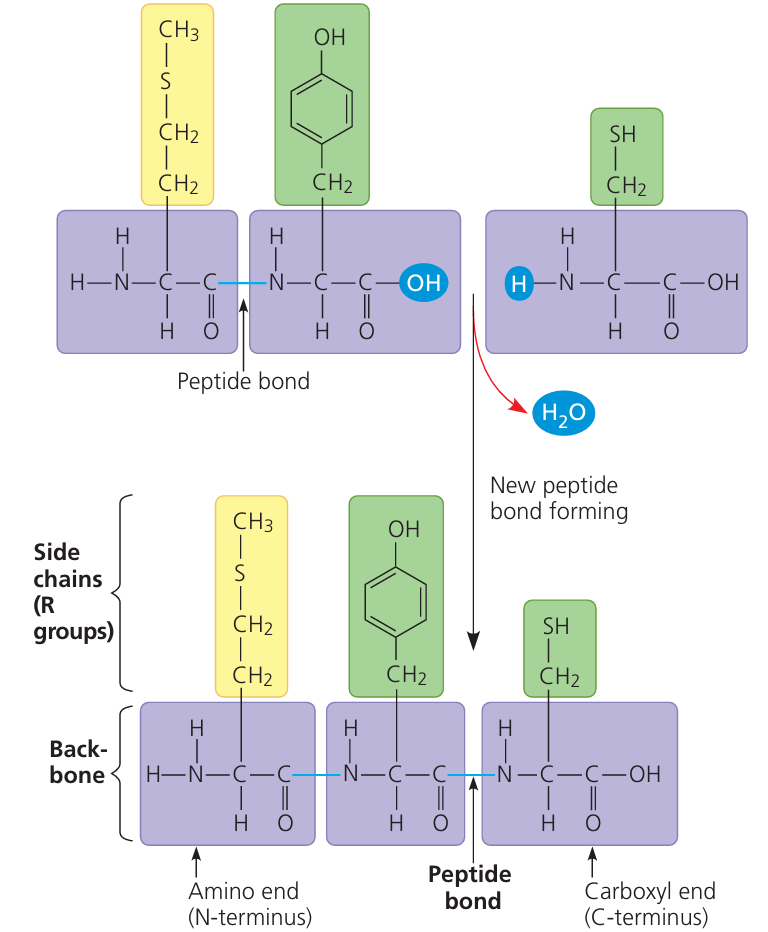
- Proteins are polymers made of amino acid monomers.
- These amino acids are linked together in a specific sequence to form polypeptide chains.
🔗 Peptide Bond Formation
A peptide bond is a covalent bond that connects:
- The carboxyl group (–COOH) of one amino acid
- to the amino group (–NH2) of the next amino acid
This reaction is a dehydration synthesis (removal of water).
🧪 Example:
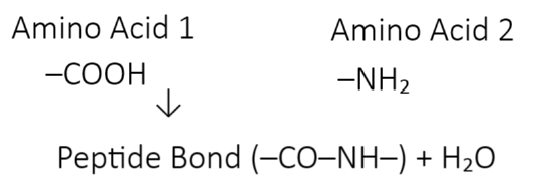
📈 What Happens Next?
- As more amino acids are added, the polypeptide chain grows.
- This chain folds into a functional 3D protein (covered in later sections).
🧠 Why It Matters
Proteins are essential for structure and function in living organisms:
- Enzymes
- Hormones
- Antibodies
- Transport proteins
The order and type of amino acids determines a protein’s final shape and function.
1.7.A.2 – Structure of Amino Acids & Role of R Groups
🧱 Basic Structure of an Amino Acid
Every amino acid has the same core structure:
- A central carbon (C) atom
- Bonded to:
- One hydrogen atom (H)
- One carboxyl group (–COOH)
- One amino group (–NH2)
- One variable R group (side chain)
🧪 Think of it like a base + a unique “flavor” (the R group).
R Group = What Makes Each Amino Acid Unique
The R group (side chain) determines the amino acids:
- Chemical behavior
- Shape and function of the protein region
🔍 R Group Categories:
| Type | Behavior | Example Trait |
| Nonpolar (Hydrophobic) | Repels water, folds inward | Found inside proteins |
| Polar (Hydrophilic) | Attracts water, folds outward | Found on surfaces |
| Ionic (Charged) | Can form ionic bonds or salt bridges | Helps with structure |
🌀 Why R Groups Matter in Proteins
Interactions between R groups (like hydrogen bonds, ionic bonds, and hydrophobic interactions) drive the protein’s:
- Folding
- 3D shape
- Function
🧬 A single amino acid change in the R group → can change the entire protein’s behavior (e.g., sickle-cell anemia).
✅ Quick Recap:
- Same amino acid backbone
- Different R groups → Different properties
- R group interactions = protein structure + function
1.7.A.3 – Protein Structure: How Sequence Shapes Function
🔗 Primary Structure = Amino Acid Sequence
The primary structure of a protein is the exact order of amino acids in a polypeptide chain.
This sequence is unique to each protein and is like a biological sentence written in amino acid “letters.”
✍️ Think of it as:
Ala–Gly–Ser–Leu–Val–… (like spelling out a word)
🧩 Shape Depends on Sequence
Even a small change in one amino acid can affect:
- How the protein folds
- Its shape
- Its function
💥 Example: One amino acid change in hemoglobin → causes sickle-cell disease.
🔁 How Sequence Leads to Shape:
Primary Structure: The linear chain of amino acids
- This sequence determines the:
- Secondary structure (α-helices, β-sheets)
- Tertiary structure (3D folding)
- Quaternary structure (if multiple chains come together)
📌 Each level builds on the one before it – and the primary sequence is the foundation.
✅ Quick Recap:
- Amino acid sequence = primary structure
- Sequence controls folding and shape
- Shape controls function
- Even one amino acid change can affect the entire protein!
1.7.A.4 – Protein Secondary Structure
🧩 What is Secondary Structure?
- Secondary structure is the local folding of a protein’s amino acid chain into specific patterns.
- This folding happens due to hydrogen bonds between atoms in the polypeptide backbone (not the R-groups yet!).
🔄 Two Common Shapes:
Alpha Helix (α-helix)
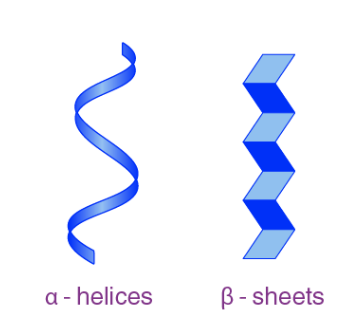
- A coiled, spiral shape (like a spring)
- Stabilized by hydrogen bonds every 4 amino acids
- Found in hair, keratin, and other structural proteins
Beta Pleated Sheet (β-sheet)
- A zig-zag or folded sheet structure
- Can run parallel or antiparallel
- Found in silk and many enzymes
🔗 Key Point:
These shapes form automatically due to chemical properties of the backbone and hydrogen bonding.
No R-group interaction yet – that comes in the tertiary structure!
✅ Quick Summary:
- Secondary structure = local folding (α-helix or β-sheet)
- Caused by hydrogen bonds in the backbone
- Helps build the protein’s 3D shape
1.7.A.5 – Tertiary Structure of Proteins
📦 What is Tertiary Structure?
- Tertiary structure is the overall 3D shape of a single polypeptide chain.
- It’s formed when R-groups (side chains) of amino acids interact with each other.
🔗 Types of R-Group Interactions (These hold the 3D shape):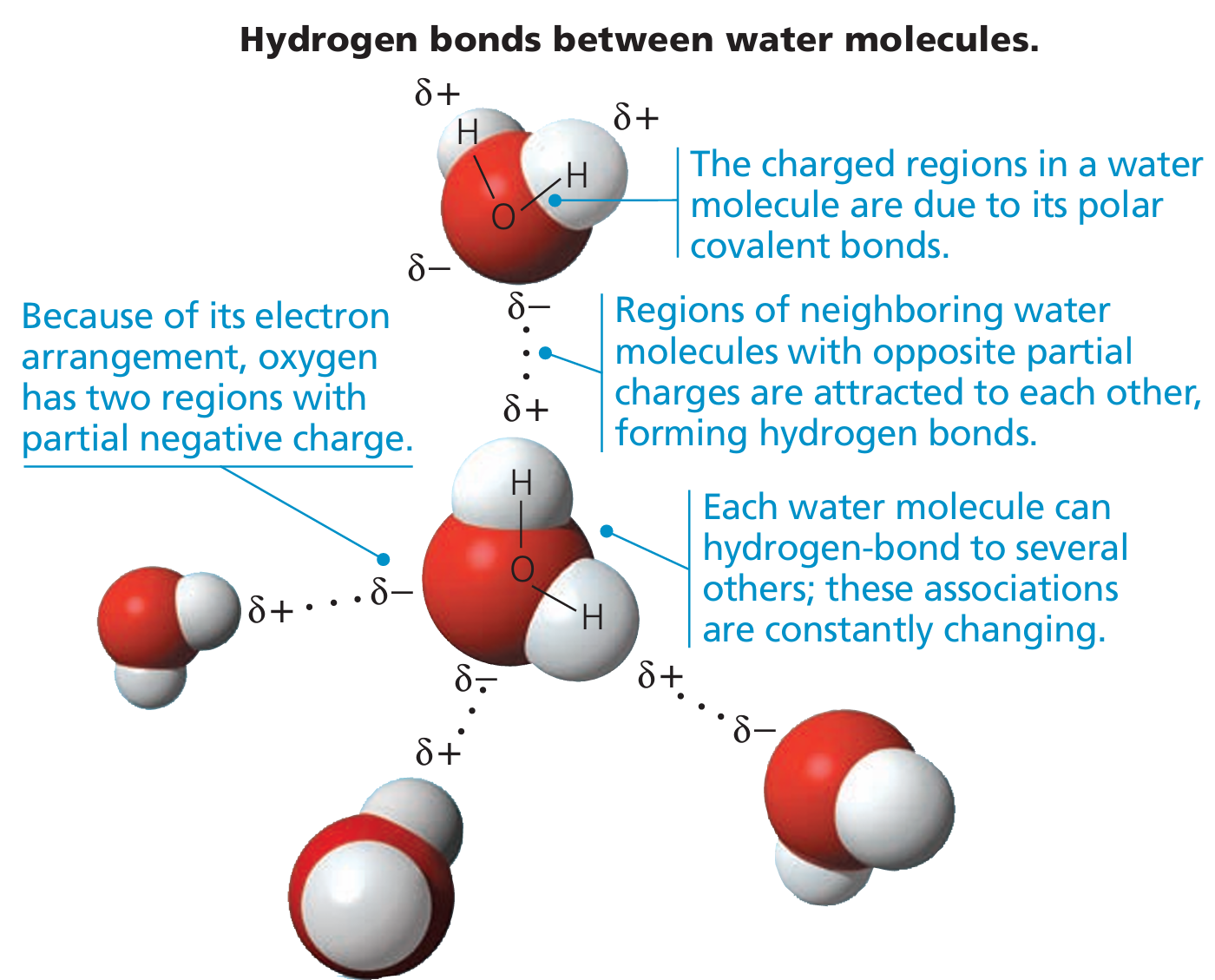
- Hydrogen Bonds
- Between polar R-groups
- Weak but stabilizing
- Ionic Bonds
- Between positively and negatively charged R-groups (acidic/basic)
- Hydrophobic Interactions
- Nonpolar R-groups clump together inside the protein, away from water
- Disulfide Bridges (Covalent Bond)
- Between sulfur atoms in cysteine R-groups
- Very strong bond, adds stability
🌀 Why It Matters:
Tertiary structure determines protein function – shape = function!
One wrong interaction → misfolded protein → possible diseases (e.g., sickle-cell anemia)
✅ Quick Summary:
- Tertiary = full 3D shape of the polypeptide
- Caused by interactions between R-groups
- Held together by hydrogen, ionic, hydrophobic, and disulfide bonds
1.7.A.6 – Quaternary Structure of Proteins
🔄 What is Quaternary Structure?
- Quaternary structure is the final level of protein structure.
- It forms when two or more polypeptide chains (called subunits) join together.
These subunits are held together by the same types of interactions found in tertiary structure: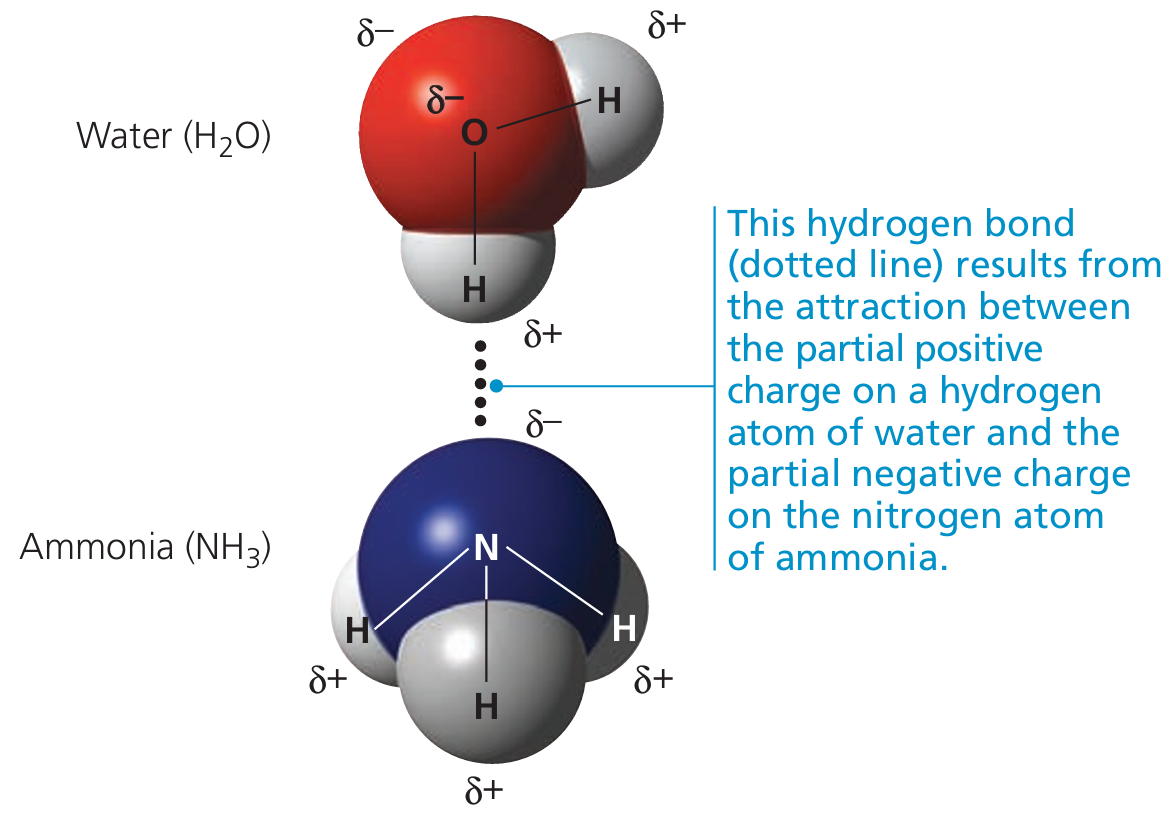
- Hydrogen bonds
- Ionic bonds
- Hydrophobic interactions
- Disulfide bridges
🧩 Example:
Hemoglobin
- Has 4 subunits (2 alpha + 2 beta chains)

- All work together to transport oxygen
🔍 All Four Levels Matter:
- Primary – amino acid sequence
- Secondary – alpha helices & beta sheets
- Tertiary – 3D folding of a single chain
- Quaternary – multiple chains working as one protein
🧠 If any level is disrupted → the protein may not function properly.
✅ Quick Recap:
- Quaternary structure = multiple folded polypeptides coming together
- Final shape = final function
- Not all proteins have this level, but when they do — it’s essential for their activity