AP Biology 2.7 Tonicity and Osmoregulation Study Notes - New Syllabus Effective 2025
AP Biology 2.7 Tonicity and Osmoregulation Study Notes- New syllabus
AP Biology 2.7 Tonicity and Osmoregulation Study Notes – AP Biology – per latest AP Biology Syllabus.
LEARNING OBJECTIVE
2.7.A : Explain how the structure of biological membranes influences selective permeability.
2.7.B : Explain how osmoregulatory mechanisms contribute to the health and survival of organisms.
Key Concepts:
- Tonicity and Osmoregulation
2.7.A – How Concentration Gradients Affect Molecular Movement
Concentration gradients drive the direction and type of movement across membranes.
📉 What Is a Concentration Gradient?
A difference in concentration of a substance across a space or membrane
- One side = high
- Other side = low
🔄 How Molecules Move
| Type of Movement | Energy? | Direction | Example |
| Passive transport | ❌ No | High → Low | O₂, CO₂, glucose, water |
| Facilitated diffusion | ❌ No | High → Low (with help) | Glucose via carrier proteins |
| Active transport | ✅ Yes | Low → High (against gradient) | Na⁺ pumped out of cells |
🧪 Why It Matters
Gradients determine:
- Which way things move
- How fast they move
- Whether energy is needed
✅ Summary
- Concentration gradients are the driving force behind molecular movement.
- Molecules move from high to low (passively) unless energy is used to go the other way (actively).
2.7.A.1 – Water Movement, Tonicity & Water Potential
Water moves by osmosis from areas of high to low water potential, depending on the tonicity of environments.
🌊 Tonicity = Relative Solute Concentration
| Term | Solute Level | Water Movement Direction | Effect on Cell 🧫 |
|---|---|---|---|
| Hypotonic | Low outside | Water goes into cell | Cell swells or bursts (lysis in animal) |
| Hypertonic | High outside | Water moves out of cell | Cell shrinks (crenates or plasmolyzes) |
| Isotonic | Equal | No net water movement | Cell stays the same ⚖️ |
🧪 Water Moves by Osmosis Water moves from:
Water moves from:
High water potential → Low water potential
OR: Hypotonic → Hypertonic solution
🧮 Water Potential Formula
\[ \Psi = \Psi_p + \Psi_s \]
| Symbol | Meaning |
|---|---|
| \(\Psi\) | Total water potential |
| \(\Psi_p\) | Pressure potential (turgor pressure) |
| \(\Psi_s\) | Solute potential (more solute → lower \(\Psi_s\)) |
 More solute = more negative \(\Psi_s\) = lower total water potential \(\Psi\).
More solute = more negative \(\Psi_s\) = lower total water potential \(\Psi\).
✅ Summary
Water moves toward areas with more solute (lower water potential).
This is how cells respond to hypotonic, hypertonic, or isotonic surroundings to maintain balance and prevent damage.
2.7.B – Osmoregulation & Survival
Organisms use osmoregulatory mechanisms to maintain water balance – essential for life and health.
🌊 What Is Osmoregulation?
Osmoregulation = controlling water and solute levels to keep internal balance
Prevents:
- 💧 Too much water intake (cell bursting)
- 🧂 Too much solute or water loss (cell shrinking)
🧬 Why It’s Important
Cells must stay in the right water environment to:
- Keep shape
- Allow enzyme function
- Avoid lysis (bursting) or plasmolysis (shrinking)
🧍 Examples of Osmoregulatory Mechanisms
| Organism | Strategy Used |
|---|---|
| Freshwater protists | Use contractile vacuoles to pump out excess water 🌊 |
| Marine fish | Drink seawater & excrete salt through gills 🐠 |
| Humans | Kidneys regulate water and ion balance via urine concentration 🚽 |
| Plants | Use guard cells to control water loss via stomata 🌿 |
✅ Summary
Osmoregulatory mechanisms help organisms survive in different environments by keeping water and solute levels stable. This protects cells from damage and supports overall health and function.
2.7.B.1 – Membrane Transport Supports Growth & Homeostasis
Continuous movement of molecules across membranes keeps cells healthy and growing.
🧬 Why Constant Movement Matters
Cells are not static they’re always taking in and sending out materials!
Growth needs:
- Nutrients (glucose, amino acids) in
- Waste products out
- Water balance maintained
⚖️ Homeostasis Depends on:
| Transport Process | Role in Cell Health |
|---|---|
| Passive transport | Maintains balance (e.g., O₂ in, CO₂ out) |
| Active transport | Moves ions to maintain internal conditions |
| Osmosis | Regulates water flow to prevent bursting/shrinking |
| Endo/Exocytosis | Moves large molecules in/out (e.g., hormones, waste) |
✅ Summary
To grow, respond to the environment, and maintain homeostasis, cells constantly move molecules across membranes using various transport mechanisms.
2.7.B.2 – Osmoregulation & Water Movement
Osmoregulation keeps water and solute levels balanced by controlling water movement based on solute concentration.
🌊 How Water Moves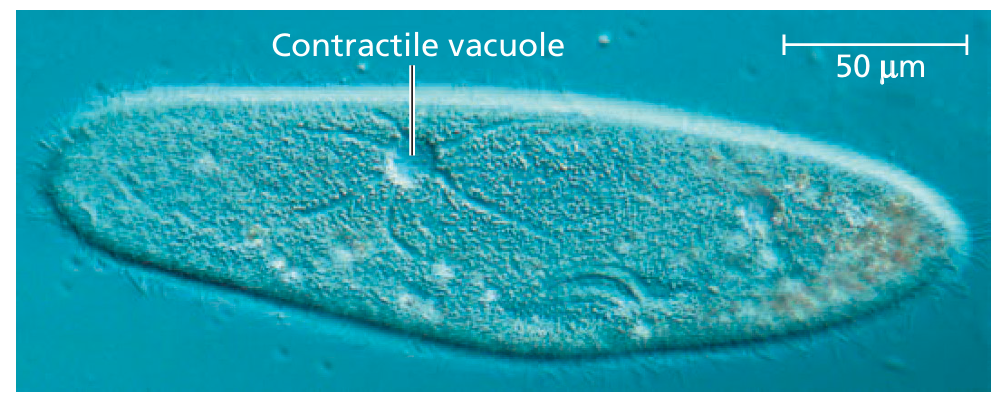
Water flows by osmosis:
- From low solute concentration (low osmolarity)
- To high solute concentration (high osmolarity)
This helps organisms balance:
- 🧂 Solute levels
- 💧 Water content
🧪 Osmoregulation = Balance
| Role | Why It Matters |
|---|---|
| Controls solute composition | Prevents toxic build-up or deficiency |
| Maintains water potential | Ensures proper water flow in/out |
| Supports cell turgor (plants) | Keeps cells firm, prevents wilting |
🧮 Solute Potential Equation
\[ \Psi_s = -iCRT \]
| Symbol | Meaning |
|---|---|
| i | Ionization constant (e.g., NaCl = 2) |
| C | Molar concentration (mol/L) |
| R | Pressure constant = 0.0831 L·bars/mol·K |
| T | Temperature in Kelvin (°C + 273) |
More solute = more negative \(\Psi_s\) → Lower water potential → Water moves in.
✅ Summary
- Osmoregulation helps organisms control water and solute levels by influencing water potential.
- Water moves from low to high solute areas, and this movement can be predicted using the −iCRT equation.
