AP Biology 3.2 Environmental Impacts on Enzyme Function Study Notes - New Syllabus Effective 2025
AP Biology 3.2 Environmental Impacts on Enzyme Function Study Notes- New syllabus
AP Biology 3.2 Environmental Impacts on Enzyme Function Study Notes – AP Biology – per latest AP Biology Syllabus.
LEARNING OBJECTIVE
Explain how changes to the structure of an enzyme may affect its function.
Key Concepts:
- Environmental Impacts on Enzyme Function
3.2.A – Environmental Impacts on Enzyme Function
🔧 Enzyme Shape = Enzyme Function

Enzymes are proteins, and their 3D shape (structure) is crucial for how they work.
- The active site is the specific part where the substrate binds.
- If the enzyme’s shape changes, especially at the active site, the substrate may no longer fit — and the enzyme won’t function properly.
⚠️ What Causes Structural Changes?
Structural changes in enzymes often result from environmental factors:
🌡️ Temperature
- Too cold: enzyme activity slows down (less kinetic energy).
- Too hot: enzyme may denature (lose shape) and stop working.
📈 pH
- Each enzyme has an optimal pH range.
- Too acidic or too basic = disrupts bonds → enzyme may denature.
🧂 Salt Concentration (Ionic Conditions)
- Too much or too little salt can interfere with ionic bonds → affects structure.
❌ Denaturation = Enzyme Breakdown
- Denatured enzymes have permanently altered shapes.
- The active site becomes distorted → substrate can’t bind.
- Denatured enzymes cannot regain their original structure or function.
🧠 Summary Table:
| Factor | Effect on Enzyme |
|---|---|
| Temperature ↑↓ | Too high → denatures Too low → slows down activity |
| pH changes | May denature or reduce activity |
| Ionic concentration | Disrupts bonding, may change structure |
👉 Key Concept: Structure determines function – if the enzyme’s structure changes, its function is affected!
3.2.A.1 – How the Environment Affects Enzyme Function
🧬 1. Enzyme Structure = Enzyme Efficiency
- Enzymes are proteins, and their function depends on their shape.
- A change in structure (shape) can either:
- Reduce efficiency
- Stop the enzyme from working completely
🔥 2. Denaturation = Breakdown of Enzyme Structure
- Denaturation occurs when an enzyme loses its shape.
- It can no longer bind to its substrate → reaction cannot occur.
🧪 Causes of Denaturation:
- High temperatures
- Extreme pH (too acidic or too basic)
- Harsh chemical environments (e.g., alcohol, heavy metals)
🔁 Denaturation is usually irreversible – enzyme function is lost permanently.
🌡️ 3. Outside the Optimal Range = Trouble
- Each enzyme has an optimal:
- Temperature
- pH
- Outside these ranges can cause:
- Disruption of hydrogen bonds
- Change in enzyme shape
- ⬇️ Lower catalytic efficiency (enzyme slows or stops)
🧠 Quick Recap Table:
| 🔧 Factor | ⚠️ Effect on Enzyme |
|---|---|
| High Temp | Denatures enzyme (shape breaks → can’t function) |
| Low Temp | Slows reaction (less kinetic energy) |
| Wrong pH | Breaks hydrogen bonds → active site shape changes |
| Harsh Chemicals | Denature enzyme or interfere with binding |
👉 Key Idea: If you mess with an enzyme’s environment, you mess with its structure. And if you mess with its structure, you mess with its function.
3.2.A.2 – Reversible Denaturation of Enzymes
🧬 Can Enzymes “Bounce Back”?
Denaturation = Loss of structure → loss of function.
But not always permanent!
🔁 In some cases, if the damaging condition is removed quickly and mildly, the enzyme can refold into its original shape and regain activity.
✅ When Reversal is Possible:
- Mild temperature change (e.g., slight heating)
- Small pH shifts
- Temporary chemical stress
If these changes are not too extreme, the hydrogen bonds and interactions can reform, and the enzyme becomes functional again.
❌ When Reversal is NOT Possible:
- High heat
- Strong acids or bases
These usually cause permanent denaturation. The protein unfolds too much → cannot refold properly → enzyme is permanently inactive.
🧠 Key Point:
Not all denaturation is a death sentence for enzymes some can recover if the environment returns to normal in time.
3.2.B – How the Cellular Environment Affects Enzyme Activity
🧬 Enzymes Don’t Work in Isolation
Enzyme activity is highly sensitive to the cellular environment –
even small changes can boost or block how well they work!
🌡️ 1. Temperature
- Every enzyme has an optimal temperature
- Too low: Particles move slowly → fewer collisions → reaction slows down
- Too high: Enzyme may denature → shape is lost → reaction stops
⚖️ 2. pH Levels
- Each enzyme works best at a specific pH
- Example:
- Pepsin (stomach) → best at acidic pH
- Trypsin (intestine) → best at basic pH
- Changes in pH disrupt hydrogen bonds → enzyme denatures
🧂 3. Salt Concentration / Ionic Conditions
- Too many or too few ions (Na⁺, K⁺, etc.) affect:
- Shape of the active site
- Charge interactions between enzyme & substrate
- Extreme ionic changes → denaturation or reduced binding
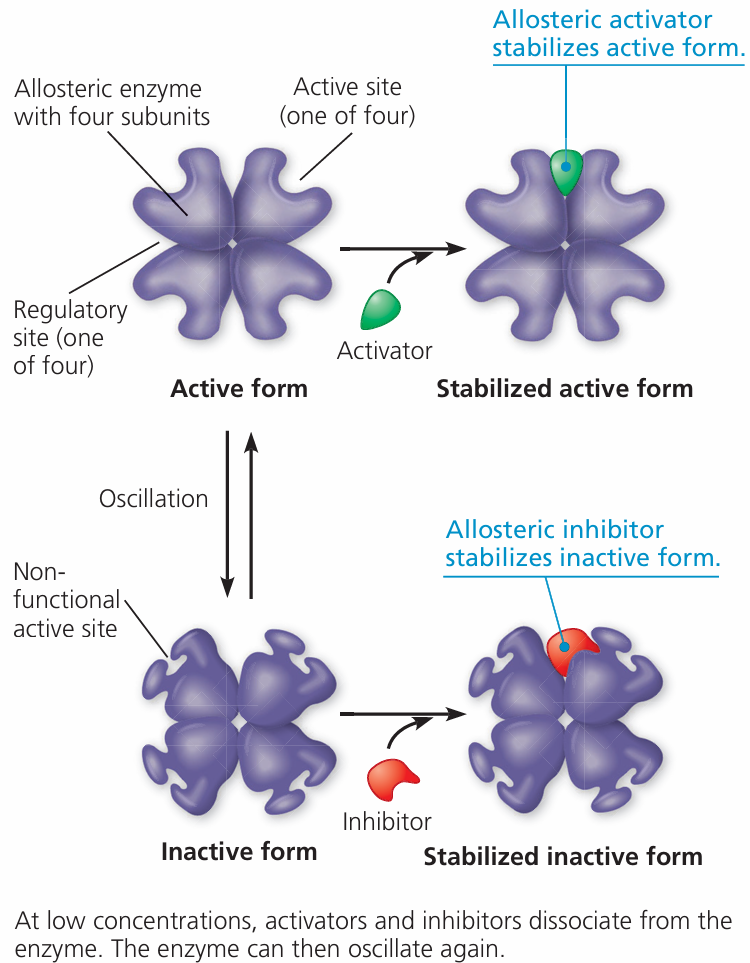
🧪 4. Presence of Inhibitors or Activators
- Inhibitors: Slow or stop enzyme activity (e.g., competitive or noncompetitive)
- Activators: Molecules that help enzymes work better/faster
📌 Key Point:
Enzyme activity depends on a stable, optimal cellular environment.
Any major change in temperature, pH, ion levels, or chemical presence can affect how well enzymes catalyze reactions.
3.2.B.1 – Substrate & Product Concentration Effects on Enzyme Activity
⚖️ How Concentration Influences Enzyme Efficiency
Enzymes don’t work in a vacuum — their activity depends on the amount of substrate available and the buildup of products during a reaction.
🔹 1. Substrate Concentration (How much starting material there is)
- Low substrate: Slower reaction (fewer enzyme-substrate collisions)
- Increasing substrate: Increases reaction rate 🟢
- Vmax: Once all enzyme active sites are full (saturation), the rate plateaus — this is the maximum velocity
🔹 2. Product Concentration (How much is already made)
- High product levels: Can slow down reactions due to feedback inhibition
- Sometimes the product binds to the enzyme → reduces its activity ❌
📌 Summary Key Idea:
The balance of substrates and products controls enzyme activity.
Too little substrate = slower reaction; too much product = slowdown due to feedback inhibition.
3.2.B.2 – Temperature Effects on Enzyme Activity
🔥 How Temperature Affects Enzymes
Enzymes are highly sensitive to heat – temperature changes can speed them up, slow them down, or even destroy their shape if too extreme.
🔹 1. Higher Temperature = Faster Molecules
- Heat increases the kinetic energy of molecules → they move faster
- Faster movement = more collisions between enzymes and substrates
- Result: Increased reaction rate ⚡
🔹 2. Optimal Temperature = Peak Efficiency
- Every enzyme has an optimal temperature 🧬
- At this temp, the enzyme is perfectly shaped and works at maximum speed
🔹 3. Too Hot? = Denaturation Risk
- Above the optimal temp → enzyme begins to denature
- The active site changes shape → substrate no longer fits
- Result: Reaction rate drops 🔻
🧠 Key Idea:
Temperature boosts enzyme activity – but only up to a point. If it gets too hot, the enzyme’s structure breaks down and its function is lost.
3.2.B.3 – Competitive vs. Noncompetitive Inhibition
🔐 What Are Inhibitors?
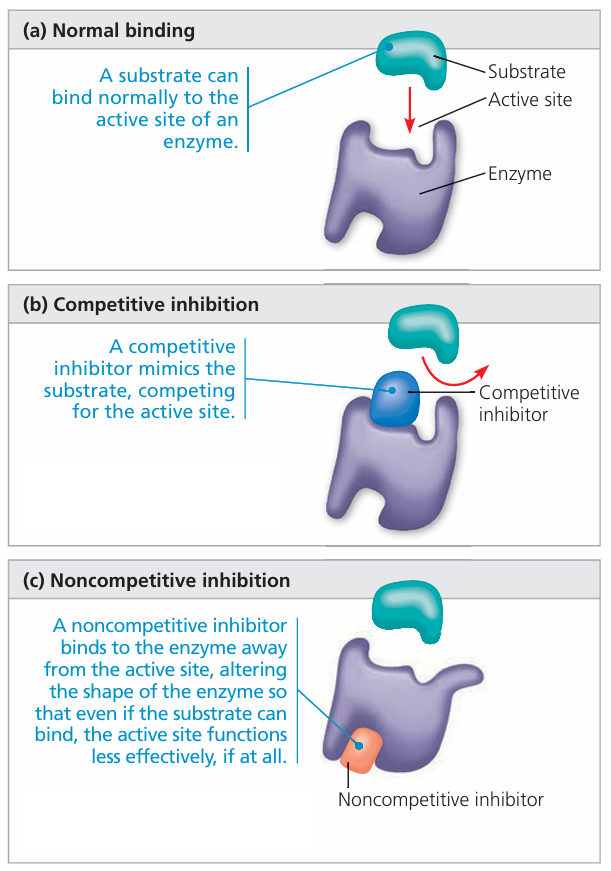
Inhibitors are molecules that slow down or stop enzyme activity by interfering with how substrates bind to the enzyme.
🆚 Two Types of Inhibitors:
🟡 1. Competitive Inhibitors
- Mimic the substrate and bind directly to the active site.
- They compete with the actual substrate for the same binding spot.
- Reversible – they can detach, allowing the real substrate to bind again.
- Adding more substrate can outcompete the inhibitor and restore activity.
🔵 2. Noncompetitive Inhibitors
- Bind to an allosteric site (not the active site).
- Cause the enzyme to change shape, altering or disabling the active site.
- Substrate cannot bind effectively → reaction slows or stops.
- Adding more substrate won’t overcome the inhibition.
🧠 Key Idea:
Competitive = blocks the spot.
Noncompetitive = changes the shape.
