AP Biology 1.1 Structure of Water and Hydrogen Bonding Study Notes - New Syllabus Effective 2025
AP Biology 1.1 Structure of Water and Hydrogen Bonding Study Notes- New syllabus
AP Biology 1.1 Structure of Water and Hydrogen Bonding Study Notes – AP Biology – per latest AP Biology Syllabus.
LEARNING OBJECTIVE
Explain how the properties of water that result from its polarity and hydrogen bonding affect its biological function.
Key Concepts:
- Structure & Properties of Water
- Hydrogen Bonding in Water
1.1.A – Structure of Water & Hydrogen Bonding
🔬 What’s so special about water?
Water isn’t just “H₂O” – it has some really cool properties because of its structure and polarity.
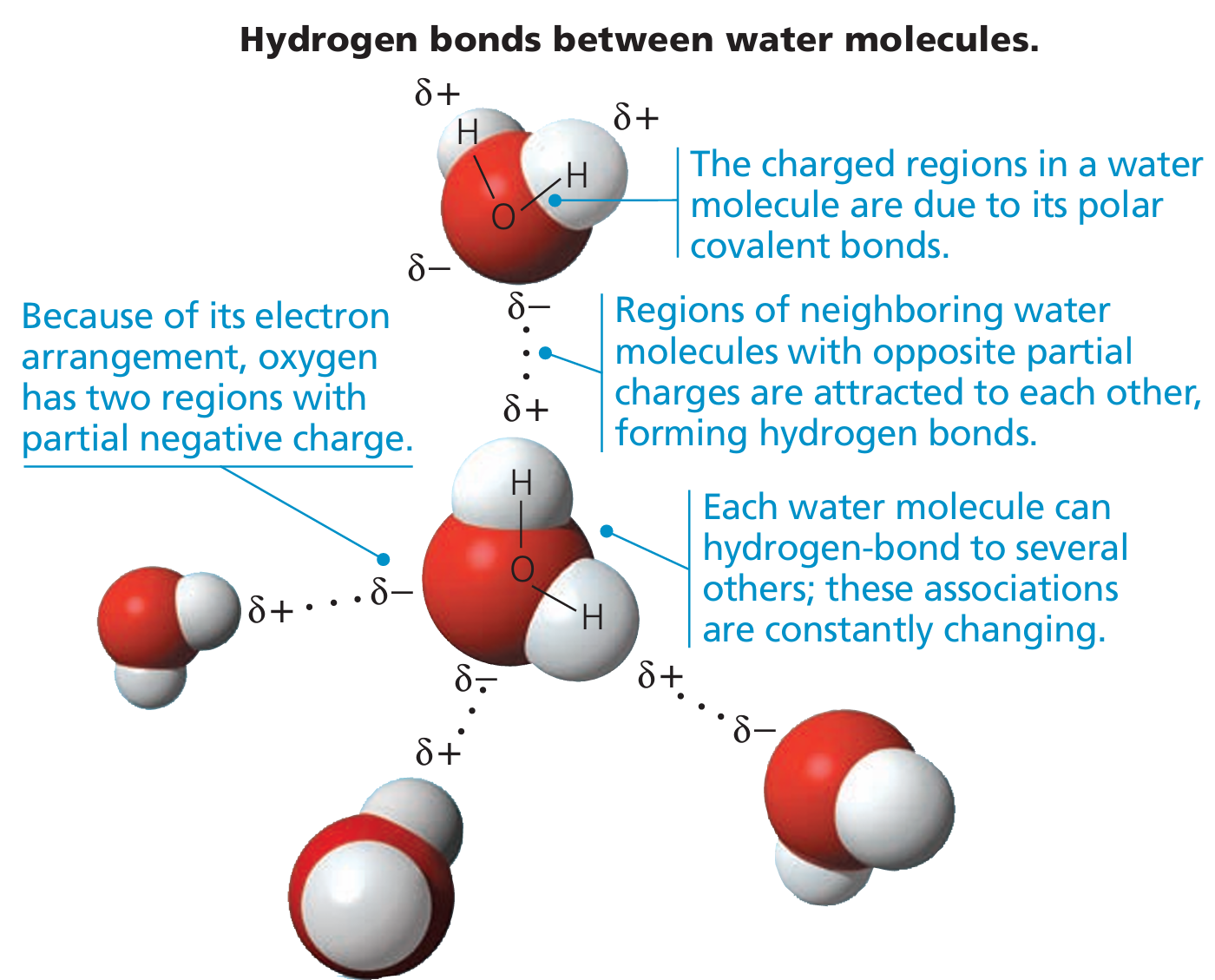
- Water is polar → One side (oxygen) is slightly negative, and the other side (hydrogens) are slightly positive.
- Because of this, water molecules stick to each other using weak attractions called hydrogen bonds.
💡 Why hydrogen bonding matters:
| Property | What it means | Why it matters for life |
|---|---|---|
| Cohesion | Water sticks to water | Helps water move up plant stems (capillary action) |
| Adhesion | Water sticks to other things | Helps water “climb” surfaces (e.g., xylem walls) |
| High specific heat | Takes a lot of energy to change temp | Stabilizes temperature in cells and ecosystems |
| High heat of vapor. | Water takes energy when it evaporates | Keeps organisms cool when sweating or transpiring |
| Ice floats | Ice is less dense than liquid water | Lakes don’t freeze from the bottom – life survives |
| Universal solvent | Can dissolve many substances | Essential for chemical reactions and nutrient transport |
🧠 Real-life examples:
- Your body stays cool when you sweat → water carries heat away
- Plants don’t need pumps → water moves up using cohesion + adhesion
- Oceans don’t boil or freeze easily → Earth’s climate is stable
- Cells are full of water → all reactions happen in a watery environment
🔁 Summary:
- Water’s polarity allows hydrogen bonds, which give water its unique properties.
- These make it perfect for life regulating temperature, moving nutrients, supporting cells, and more.
1.1.A.1 – Why Water Is So Important for Life
Living things (including us!) completely depend on water – not just to drink it, but because its special chemical properties help keep cells and body systems stable.
Let’s break down why water is such a lifesaver:
💧 1. Water is polar – it has “charged ends”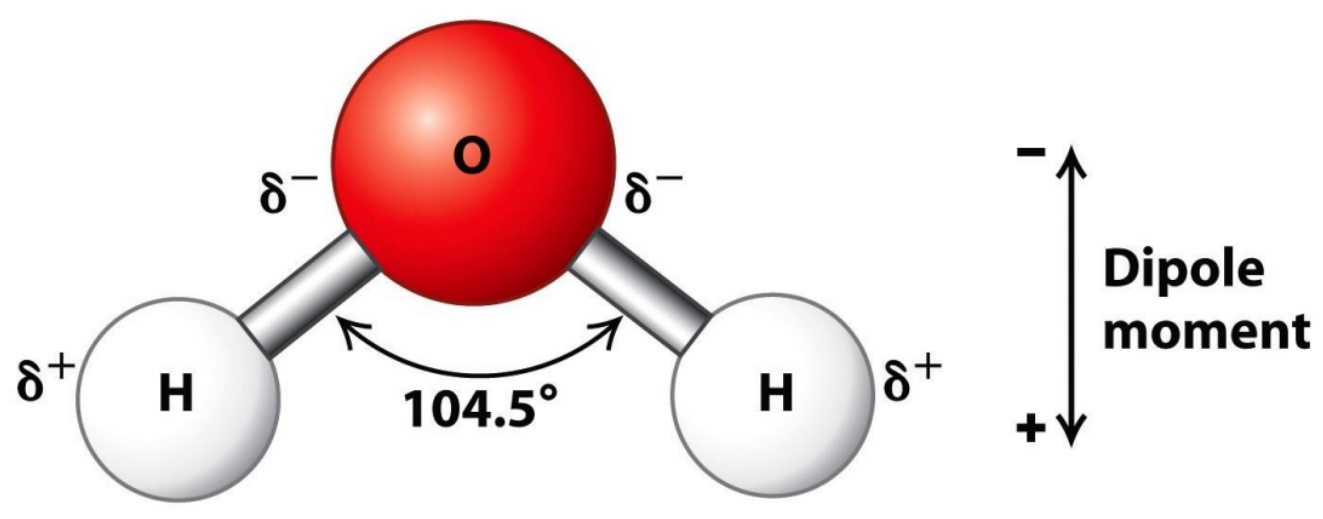
Water molecules have one oxygen atom and two hydrogens.
Because oxygen pulls electrons more strongly, it becomes slightly negative, while the hydrogens are slightly positive.
This makes the whole molecule polar, and because of this…
- Hydrogen bonds can form between water molecules and also with other molecules like DNA or proteins.
- These hydrogen bonds help stabilize biological structures.
🌡️ 2. Water has a high specific heat (doesn’t heat up easily)
- It takes a lot of energy to change the temperature of water.
- This helps organisms maintain a stable body temperature – even when the environment changes.
- Great for homeostasis! Your body won’t overheat too fast.
💨 3. Water also has a high heat of vaporization
- When water evaporates (like sweat), it takes a lot of heat with it.
- This is why sweating cools you down – heat leaves your body with the water.
- Very useful for thermoregulation in living organisms.
❄️ 4. Ice floats – weird but important!
- Most substances get denser when they freeze, but not water.
- When water freezes, hydrogen bonds lock the molecules into a structure that takes up more space (making it less dense).
- So, ice floats on liquid water.
- This is super important for aquatic life: lakes freeze from the top, not bottom – life can still survive underneath during winter.
🧠 Summary:
- Water is no ordinary liquid. Its polarity and hydrogen bonding give it unique properties like high specific heat, evaporative cooling, and floating ice.
- All these are critical for life – from keeping your body cool to allowing life to exist in lakes in winter.
1.1.A.2 – Hydrogen Bonds: Cohesion, Adhesion & Surface Tension
Water isn’t just “wet” – it behaves in amazing ways thanks to hydrogen bonding between its molecules. These bonds are weak individually, but very strong together, and they give rise to 3 key properties: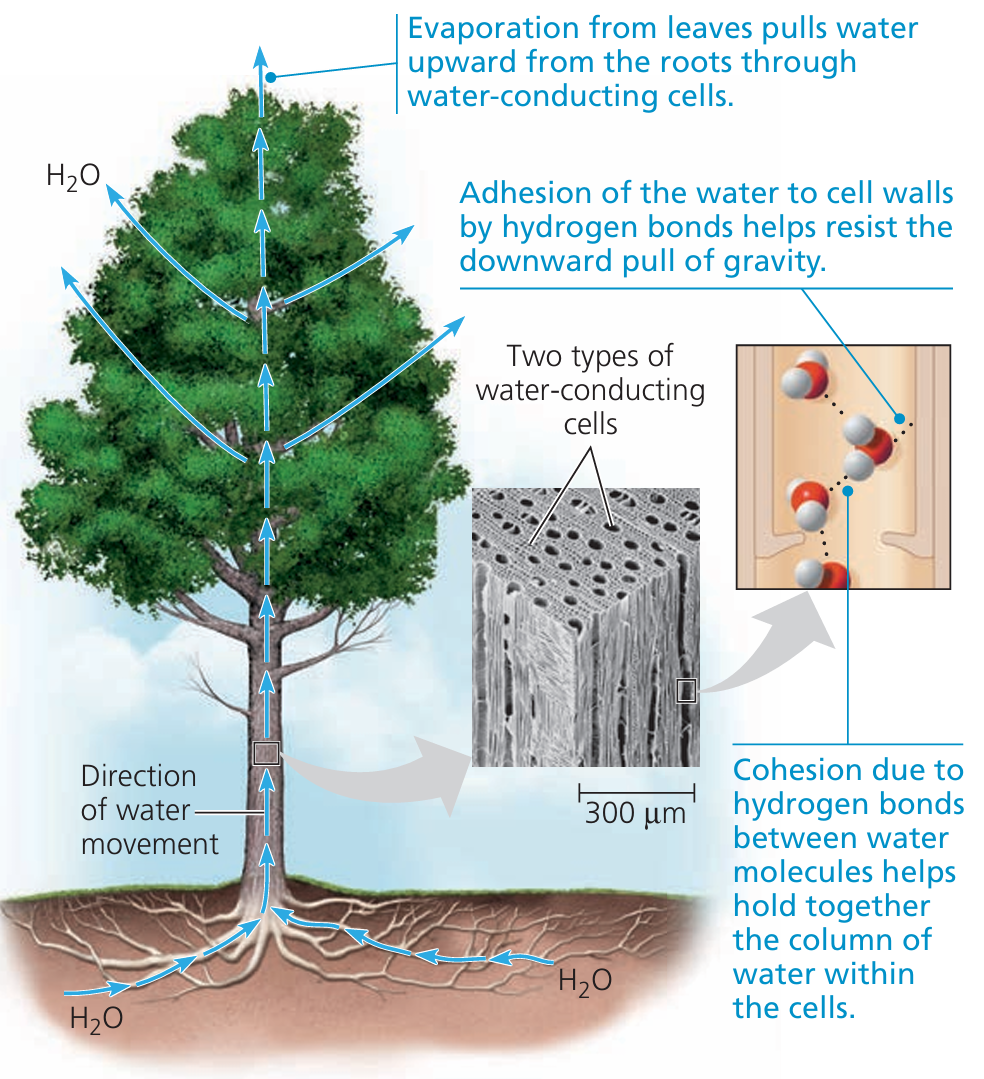
💙 1. Cohesion – water sticks to itself
- This is the tendency of water molecules to cling together.
- Happens because hydrogen bonds hold water molecules together.
- 🌿 Real-life example: During transpiration in plants, as water evaporates from the leaves, it pulls up other water molecules through the plant – like a long chain from root to leaf.
💧 2. Adhesion – water sticks to other things
- Water molecules also stick to other polar or charged surfaces.
- Helps water cling to the walls of plant vessels.
- When combined with cohesion, this enables capillary action – water can move upward in narrow tubes (like inside plant stems).
🌊 3. Surface Tension – water resists breaking at the surface
Because of cohesion, water molecules at the surface form a tight layer, almost like a “skin.”
- This allows small insects (like water striders) to walk on water without sinking.
- It also helps water form droplets.
🔁 Quick Recap:
| Property | What It Does | Why It Happens (Hydrogen Bond Role) | Example |
|---|---|---|---|
| Cohesion | Water sticks to itself | H-bonds hold water molecules together | Pulls water up plant stem |
| Adhesion | Water sticks to other materials | H-bonds with polar surfaces | Water clings to vessel walls |
| Surface Tension | Water surface resists stretching | Surface H-bonds pull tightly together | Water striders walk on water |
💡 Why it matters: These properties of water are critical for life processes, especially in plants and cells. Without them, transport of water, nutrients, and temperature regulation in organisms would not work.
