Types of Chemical Bonds study notes -AP Chemistry - New Syllabus 2024-2025
Types of Chemical Bonds Study Notes -AP Chemistry
AP Chemistry: Types of Chemical Bonds Study Notes. Comprehensive coverage of topics. Prepare for the AP Chemistry Exam
LEARNING OBJECTIVE
- Explain the relationship between the type of bonding and the properties of the elements participating in the bond.
Key Concepts:
- Electronegativity Trends
- Nonpolar and Polar Covalent Bonds
- Continuum Between Ionic and Covalent Bonds
- Metallic Bonding
Electronegativity Trends
Electronegativity is the tendency of an atom in a molecule to attract shared electrons toward itself. It is a fundamental factor in determining the type of bond formed between atoms.
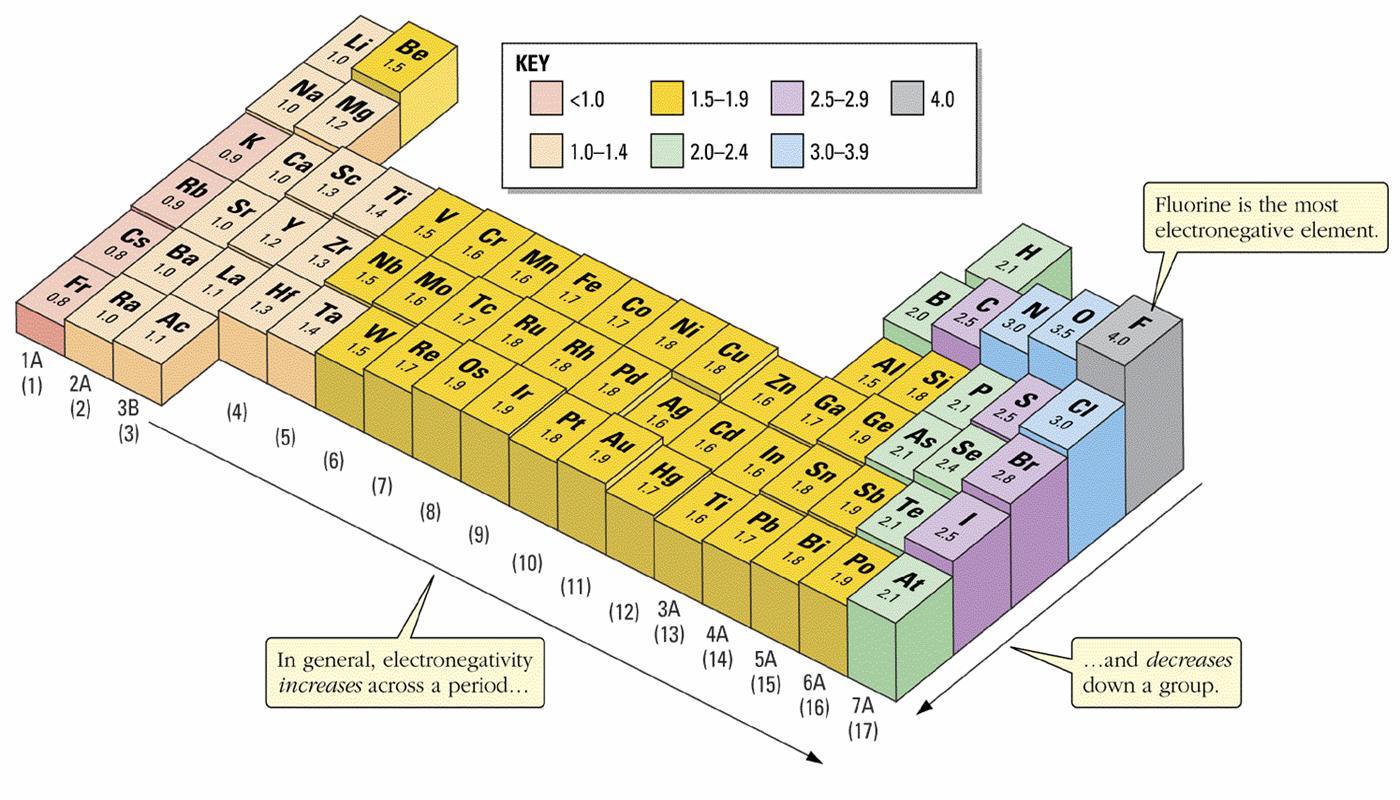
Across a Period (left → right): Electronegativity increases because:
- Effective nuclear charge increases (more protons pulling on the same shell).
- Atomic radius decreases, so electrons are held more tightly.
Down a Group (top → bottom): Electronegativity decreases because:
- Additional electron shells increase distance between nucleus and valence electrons.
- Shielding effect reduces nuclear attraction for bonding electrons.
Underlying Explanation:
- Electronic structure: Smaller atoms across a period hold electrons more tightly.
- Shell model: More shells = weaker attraction to bonding electrons.
- Coulomb’s law: The force of attraction \(\mathrm{F \propto \dfrac{q_1 q_2}{r^2}}\). As atomic radius \( \mathrm{r} \) increases, attraction for electrons decreases.
Key Idea: Electronegativity determines whether a bond will be nonpolar covalent, polar covalent, or ionic. Understanding its periodic trends helps predict bond polarity and chemical reactivity.
Example :
Compare the electronegativity of fluorine and lithium. Which is higher, and why?
▶️ Answer/Explanation
Step 1: Fluorine is to the right of lithium in the same period of the periodic table.
Step 2: Moving across a period increases effective nuclear charge and decreases atomic radius.
Step 3: Therefore, fluorine has a much higher electronegativity than lithium.
Final Answer: Fluorine’s electronegativity is higher because it has a stronger pull on bonding electrons.
Nonpolar and Polar Covalent Bonds
A covalent bond forms when two atoms share valence electrons. The distribution of electrons depends on the relative electronegativity values of the atoms.
Nonpolar Covalent Bond: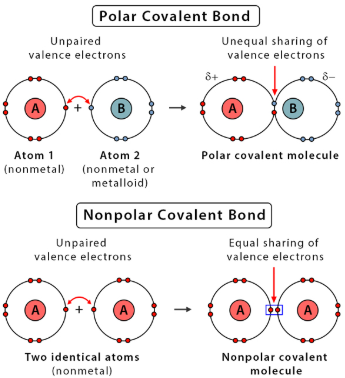
- Occurs when atoms have similar electronegativity values.
- Electrons are shared nearly equally.
- Example: \(\mathrm{C–H}\) bond (slight difference, but effectively nonpolar).
Polar Covalent Bond:
- Occurs when atoms have different electronegativity values.
- The more electronegative atom pulls shared electrons closer, creating partial charges.
- Bond dipole points toward the more electronegative atom.
Special Notes:
- The atom with higher electronegativity becomes partially negative (\(\mathrm{\delta^-}\)); the other becomes partially positive (\(\mathrm{\delta^+}\)).
- Greater electronegativity difference → stronger bond dipole.
- All polar covalent bonds have some ionic character; bonding is a continuum between pure covalent and pure ionic.
Key Idea: Bond polarity depends on electronegativity difference. Nonpolar covalent bonds lead to uniform charge distribution, while polar covalent bonds result in partial charges that strongly affect molecular properties (e.g., solubility, boiling point).
Example :
Classify the bond in each pair as nonpolar covalent, polar covalent, or ionic: (a) \(\mathrm{O–O}\) (b) \(\mathrm{H–Cl}\) (c) \(\mathrm{Na–Cl}\)
▶️ Answer/Explanation
(a) O–O: Both atoms have identical electronegativity. Electrons are shared equally → Nonpolar covalent.
(b) H–Cl: Electronegativity difference is significant (Cl is more electronegative). Electrons are unequally shared → Polar covalent.
(c) Na–Cl: Very large electronegativity difference. Electron is essentially transferred → Ionic bond.
Final Answer: (a) Nonpolar covalent, (b) Polar covalent, (c) Ionic.
Continuum Between Ionic and Covalent Bonds
Bonding is not strictly classified as “ionic” or “covalent.” Instead, it exists on a continuum, depending on the difference in electronegativity and the nature of the elements involved.
Electronegativity Difference:
- Small difference → nonpolar covalent bond.
- Moderate difference → polar covalent bond.
- Large difference → ionic bond.
General Rule:
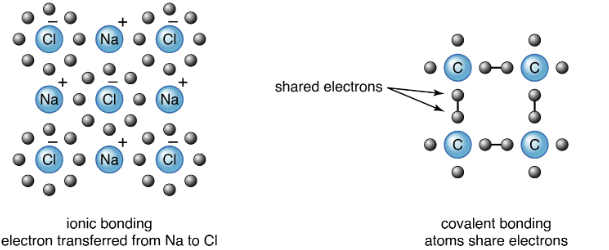
- Metal + Nonmetal → Ionic bond.
- Nonmetal + Nonmetal → Covalent bond.
Compound Properties:
- Ionic compounds: High melting points, brittle solids, conduct electricity when molten/dissolved in water.
- Covalent compounds: Lower melting points, often gases or liquids at room temperature, poor conductors.
Key Idea: The best way to characterize a bond is by examining the properties of the compound, since real bonds often show both ionic and covalent character.
Example :
Compare the bonding in \(\mathrm{NaCl}\) and \(\mathrm{H_2O}\). Explain why one is considered mostly ionic while the other is polar covalent.
▶️ Answer/Explanation
Step 1: In \(\mathrm{NaCl}\), sodium (metal) and chlorine (nonmetal) have a large electronegativity difference.
Step 2: Sodium loses an electron to form \(\mathrm{Na^+}\), and chlorine gains one to form \(\mathrm{Cl^-}\). This transfer produces a strong electrostatic attraction → ionic bond.
Step 3: In \(\mathrm{H_2O}\), hydrogen and oxygen are both nonmetals. The electronegativity difference is moderate.
Step 4: Oxygen pulls shared electrons closer, giving partial charges (\(\mathrm{\delta^-}\) on O, \(\mathrm{\delta^+}\) on H), but electrons are still shared → polar covalent bond.
Final Answer: \(\mathrm{NaCl}\) is primarily ionic, while \(\mathrm{H_2O}\) is polar covalent, illustrating the bond-type continuum.
Metallic Bonding
In a metallic solid, valence electrons from the metal atoms are not bound to individual atoms. Instead, they are delocalized, forming a “sea of electrons” that moves freely throughout the solid lattice of metal cations.
![]()
Delocalized Electrons:
- Electrons are mobile and not tied to any one atom.
- They provide strong cohesive forces that hold the lattice of positive ions together.
Physical Properties of Metals:
- Electrical conductivity: Free-moving electrons allow metals to conduct electricity.
- Thermal conductivity: Mobile electrons transfer heat efficiently.
- Malleability and ductility: Metallic bonds are nondirectional, so metal atoms can slide past each other without breaking bonds.
- Luster: Electrons can absorb and re-emit light, giving metals their shiny appearance.
Key Idea: Metallic bonding explains the unique properties of metals — high electrical and thermal conductivity, malleability, ductility, and metallic luster — due to the mobility of delocalized electrons.
Example :
Explain why copper is both a good conductor of electricity and can be drawn into wires without breaking.
▶️ Answer/Explanation
Step 1: In copper, valence electrons are delocalized and move freely through the metallic lattice.
Step 2: Free-moving electrons allow an electric current to pass easily → high electrical conductivity.
Step 3: Metallic bonds are nondirectional, meaning atoms can slide past each other without breaking bonds.
Final Answer: Copper conducts electricity well because of its delocalized electrons, and it is ductile because metallic bonds remain intact when atoms are rearranged.
