Intramolecular Force and Potential Energy study notes -AP Chemistry - New Syllabus 2024-2025
Types of Chemical Bonds Study Notes -AP Chemistry
AP Chemistry: Types of Chemical Bonds Study Notes. Comprehensive coverage of topics. Prepare for the AP Chemistry Exam
LEARNING OBJECTIVE
- Represent the relationship between potential energy and distance between atoms, based on factors that influence the interaction strength.
Key Concepts:
- Potential Energy Curve and Equilibrium Bond Length
- Covalent Bond Length and Bond Order
- Coulomb’s Law and Ionic Interactions
Potential Energy Curve and Equilibrium Bond Length
The potential energy of two atoms depends on the distance between their nuclei. A graph of potential energy versus internuclear distance shows how attractive and repulsive forces determine bond stability.
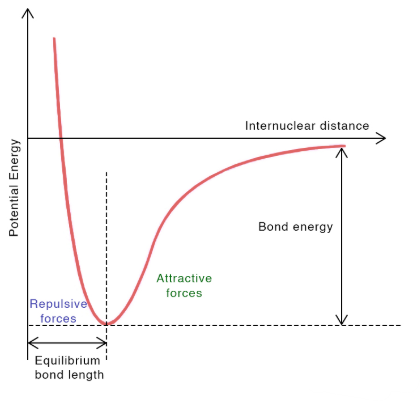
Key Properties:
- At short distances: Strong repulsion between electron clouds and nuclei causes potential energy to rise steeply.
- At intermediate distances: Attractive forces (nucleus–electron interactions) dominate, lowering potential energy.
- Equilibrium bond length: The separation at which potential energy is minimized — the most stable bond distance.
- Bond energy: The energy required to separate the atoms from equilibrium distance to infinite distance.
Key Idea: The lowest point on the potential energy curve represents the most stable arrangement of atoms, defining both bond length and bond strength.
Example :
Why is the equilibrium bond length considered the most stable arrangement of two atoms?
▶️ Answer/Explanation
Step 1: At distances shorter than the bond length, repulsive forces increase potential energy.
Step 2: At distances longer than the bond length, attractive forces weaken, and potential energy increases again.
Step 3: At the equilibrium bond length, potential energy is minimized, meaning the system is most stable.
Final Answer: The equilibrium bond length is the distance where attractive and repulsive forces balance, giving the lowest potential energy and maximum stability.
Covalent Bond Length and Bond Order
In covalent bonds, the distance between bonded atoms (bond length) and the strength of the bond (bond energy) depend on both the size of the atoms and the number of shared electron pairs (bond order).
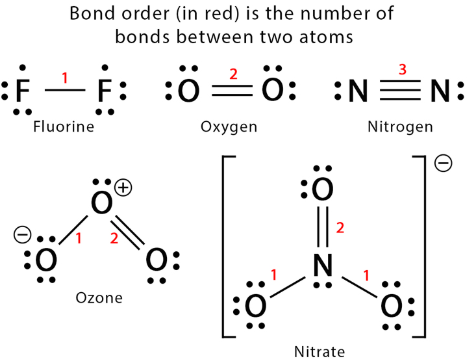
Key Properties:
- Atomic Size: Larger atoms have longer bond lengths due to greater distance between nuclei.
- Bond Order:
- Single bond: Longest and weakest.
- Double bond: Shorter and stronger.
- Triple bond: Shortest and strongest.
- Bond Energy: Higher bond order → greater electron density between nuclei → stronger attraction → larger bond energy.
Key Idea: Covalent bond length decreases and bond energy increases with higher bond order. The balance of atomic size and bond order determines the bond’s stability and strength.
Example :
Compare the bond length and bond energy of \(\mathrm{C–C}\), \(\mathrm{C=C}\), and \(\mathrm{C \equiv C}\).
▶️ Answer/Explanation
Step 1: \(\mathrm{C–C}\) is a single bond → longest bond length and lowest bond energy.
Step 2: \(\mathrm{C=C}\) is a double bond → shorter and stronger than a single bond.
Step 3: \(\mathrm{C \equiv C}\) is a triple bond → shortest bond length and highest bond energy.
Final Answer: Bond strength and bond energy increase as bond length decreases: \(\mathrm{C–C > C=C > C \equiv C}\) (length) and \(\mathrm{C–C < C=C < C \equiv C}\) (energy).
Coulomb’s Law and Ionic Interactions
The strength of ionic interactions (electrostatic attraction between cations and anions) can be understood using Coulomb’s law, which relates interaction energy to the charges of the ions and the distance between them.

Coulomb’s Law: \(\mathrm{F \propto \dfrac{q_1 q_2}{r^2}}\) where \(\mathrm{q_1}\) and \(\mathrm{q_2}\) are ionic charges and \(\mathrm{r}\) is the distance between nuclei.
- Charge Effect: Greater ionic charges → stronger electrostatic interactions.
- Distance Effect: Smaller ionic radii → shorter internuclear distance → stronger interactions.
- Lattice Energy: The potential energy required to separate a crystal into ions; larger charges and smaller ions produce higher lattice energies.
Key Idea: Stronger ionic interactions occur when ions have higher charges and smaller sizes, leading to shorter distances between nuclei and deeper potential energy wells.
Example :
Which compound has stronger ionic interactions: \(\mathrm{CaO}\) or \(\mathrm{NaCl}\)?
▶️ Answer/Explanation
Step 1: \(\mathrm{Ca^{2+}}\) and \(\mathrm{O^{2-}}\) have charges of +2 and –2, while \(\mathrm{Na^+}\) and \(\mathrm{Cl^-}\) have charges of +1 and –1.
Step 2: Larger charges in \(\mathrm{CaO}\) produce stronger Coulombic attraction.
Step 3: \(\mathrm{Ca^{2+}}\) and \(\mathrm{O^{2-}}\) are smaller than \(\mathrm{Na^+}\) and \(\mathrm{Cl^-}\), reducing internuclear distance further.
Final Answer: \(\mathrm{CaO}\) has stronger ionic interactions (higher lattice energy) than \(\mathrm{NaCl}\).
