Structure of Metals and Alloys Study Notes- AP Chemistry- Unit 2.4 -New Syllabus 2024-2025
Structure of Metals and Alloys Study Notes- AP Chemistry
Structure of Metals and Alloys Study Notes as per latest AP Chemistry Syllabus
LEARNING OBJECTIVE
- Represent a metallic solid and/or alloy using a model to show essential characteristics of the structure and interactions present in the substance.
Key Concepts:
- Metallic Bonding: Sea of Delocalized Electrons
- Interstitial Alloys: Smaller Atoms in Larger Atomic Spaces
- Substitutional Alloys: Atoms of Comparable Radius
Metallic Bonding and the Sea of Electrons
Metallic bonding is the attraction between positive metal cations and delocalized valence electrons that move freely throughout the entire solid. This is often described as a “sea of electrons” model.
![]()
Key Properties:
- Positive Ion Lattice: Metal atoms release valence electrons and become positive ions arranged in a regular, repeating lattice.
- Delocalized Electrons: Valence electrons are free to move throughout the structure, not bound to any specific atom.
Physical Properties:
- Conductivity: Free-moving electrons allow metals to conduct electricity and heat efficiently.
- Malleability/Ductility: The nondirectional metallic bonds allow metal ions to slide past each other without breaking the structure.
- Luster: Electrons absorb and re-emit light, giving metals their shiny appearance.
Key Idea: Metallic bonding is characterized by a lattice of metal cations immersed in a sea of mobile electrons, explaining the unique physical properties of metals.
Example :
Why is copper both a good conductor of electricity and malleable?
▶️ Answer/Explanation
Step 1: Copper atoms release valence electrons into the “sea of electrons.”
Step 2: These delocalized electrons move freely, allowing an electric current to flow → high electrical conductivity.
Step 3: Metallic bonds are nondirectional, so when layers of copper ions shift, the bonding remains intact → malleability.
Final Answer: Copper is conductive due to its mobile electrons and malleable because metallic bonds allow ions to move without breaking.
Interstitial Alloys
An interstitial alloy forms when smaller atoms occupy the spaces (interstices) between larger metal atoms in a metallic lattice.
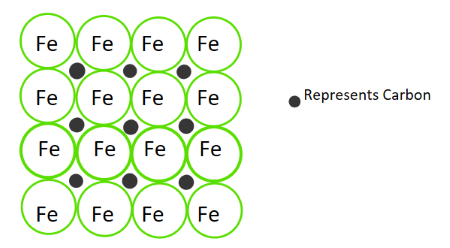
Key Properties:
- Size Difference: Occurs when atoms have very different radii.
- Structural Impact: Smaller atoms fit into the gaps, making the lattice denser and less flexible.
Effect on Properties:
- Stronger and harder than the pure metal.
- Less malleable because the presence of small atoms restricts movement of metal ions.
Key Idea: Interstitial alloys increase strength and hardness by filling spaces in the metallic lattice, reducing the ability of atoms to slide past each other.
Example :
Why is steel (iron + carbon) stronger than pure iron?
▶️ Answer/Explanation
Step 1: In steel, small carbon atoms occupy interstitial spaces between larger iron atoms.
Step 2: These carbon atoms prevent iron ions from sliding easily past each other.
Step 3: This increases the hardness and strength of the metal but decreases malleability.
Final Answer: Steel is stronger than pure iron because interstitial carbon atoms restrict ion movement in the metallic lattice.
Substitutional Alloys
A substitutional alloy forms when atoms of comparable size replace one another in the metallic lattice.
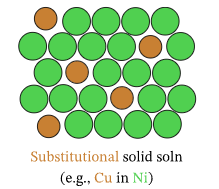
Key Properties:
- Size Similarity: Occurs when the radii of two metals are close in size.
- Structural Impact: Atoms can substitute without significantly distorting the lattice.
- Effect on Properties:
- Often improves strength compared to the pure metal.
- Retains conductivity and malleability.
Key Idea: Substitutional alloys form when one metal atom replaces another in the lattice, producing solids with altered but often balanced properties.
Example :
Why is brass (copper + zinc) harder than pure copper but still workable?
▶️ Answer/Explanation
Step 1: In brass, zinc atoms (similar size to copper) substitute for some copper atoms in the lattice.
Step 2: This substitution slightly disrupts the copper lattice, increasing hardness compared to pure copper.
Step 3: Because the size difference is small, the lattice remains largely intact, preserving malleability and conductivity.
Final Answer: Brass is harder than copper due to substitution of zinc atoms but still workable because the lattice is not significantly distorted.
