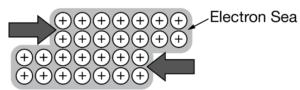
Metallic Solids
- Metallic solid: are held together by the strong forces of attraction between the positively charged metal ions and delocalized electrons (electrons not associated with a single atom or molecule)
- What accounts for differences in compound structures? The answer lies in the bonding
Comparing how Metals and Ionic Compounds Conduct Electricity
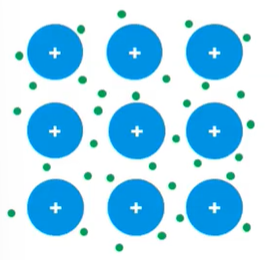
- Metals → mobile valence electrons
- Ionic compounds → mobile charged
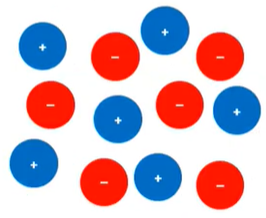 particles
particles
Properties of Metals
- Tendency to give up one or more electrons to form a positive ion → have low ionization energies
- Malleable: easy to shape/bend rather than break bcuz adjacent layers of positive metal ions can move relative to one another while remaining in full contact with the electron sea
- Ductile: pulled into wires
- Shiny: reflect light bcuz free electrons can bounce back light at the same frequency
- Solid at room temperature (except mercury which becomes liquid)
- Metals conduct electricity and heat very efficiently because of the availability of highly mobile electrons
- Metal atoms in a metallic solid generate a “sea” of mobile valence that can easily move from one atom to the next and allow electricity to flow through; Electrons are excited from filled orbitals into the very near empty orbitals
- If there is a large gap between the filled and the empty levels → electrons cannot be transferred easily to the empty conduction bands = poor conductor
- Metal atoms in a metallic solid generate a “sea” of mobile valence that can easily move from one atom to the next and allow electricity to flow through; Electrons are excited from filled orbitals into the very near empty orbitals
Metal Alloys 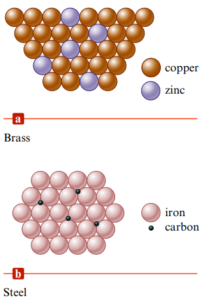
- Alloy: substance that contains a mixture of elements and has metallic properties → two types (difference is size of molecules)
- Substitutional alloy: atoms of similar sizes
- Some of the host metal atoms are replaced by another element’s of similar size
- Interstitial alloy: atoms of different sizes
- Formed when some of the holes in the metal’s structure are occupied by small atoms → presence of the interstitial atoms changes the properties of the host metal
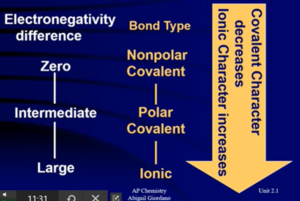
Bond Polarity and Dipole Moments
- Polarity: the difference in electronegativity (not the absolute value of electronegativity)
- Dipole/having a dipole moment: molecule that has a center of positive charge and a center of negative charge (bcuz of unequal sharing of e-) → polar molecule
- Often has arrow pointing to the (-) charge center
- Difference in electronegativity between atoms tells us how polar a compound is
- Atoms with high electronegativity with develop a partial negative charge
- Atoms with low electronegativity with develop a partial positive charge
- Any diatomic molecule that has a polar bond will have a molecular dipole moment
- With three or more atoms there are two considerations if will have a dipole moment
- There must be a polar bond & geometry cannot cancel it out
- If any of the terminal elements are different → automatically a polar molecule (polar bonds are asymmetric)
- Unbalanced pull of electrons creates a partial (-) charge on one side of central atom and partial (+) charge on other → separation of charge forms a dipole = polar
Example: HF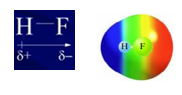
- Red indicates the most electron-rich area (the fluorine atom), and blue indicates the most electron-poor region (the hydrogen atom
Geometry and Dipole Moment
- Some molecules have polar bonds but do not have a dipole moment (are nonpolar).
- Occurs when bond dipoles are equal in magnitude and opposite in direction, canceling each other out and making the net dipole for the molecule zero
- Ex: CO2 held by polar covalent bonds but is nonpolar
- Occurs when bond dipoles are equal in magnitude and opposite in direction, canceling each other out and making the net dipole for the molecule zero
- A molecule is nonpolar when all of the bond dipoles cancel each other out
- Has equal pull of electrons and symmetrical geometry
- Molecules composed of only nonpolar bonds are nonpolar, regardless of shape
- Molecules in which the central atom is symmetrically surrounded by identical atoms are nonpolar, even if the bonds are polar
- Equal attraction of electrons → net dipole is zero
- Polar molecules may have some nonpolar bonds
- Ex: of shapes that do cancel polarity of bonds
- Ex: linear molecules with 2 identical bonds, planar molecules with 3 identical bonds 120 degrees part, tetrahedral molecules
- Ex: of shapes that do NOT cancel polarity of bonds
- Bent, trigonal pyramidal
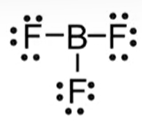 → Nonpolar: symmetrical and identical atoms
→ Nonpolar: symmetrical and identical atoms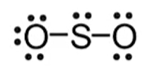 → polar: unequal share of electrons
→ polar: unequal share of electrons
Energy Effects in Binary Ionic Compounds
- Lattice energy: indicates how strong a bond is (but unlike bond energy is talking about ionic bonds)
- Determines how strongly the ions attract each other in the solid state
- Lattice energy is stronger in ionic compounds with more highly charged ions and shorter ionic distance (smaller atoms) → the one with the stronger attraction
Example Question: Out of the following pairs of compounds, pick the one w/ greater lattice energy and explain

- (2)(-2) = -4 and (1)(-1) = -1 → MgO is the correct answer
- Explain: “MgO contains the more highly charged ion and thus the ions within that compound will experience a stronger attraction”

- Have same value for charged ions, but Na is smaller than Sr and O is smaller that I → Na2O is the correct answer
- Explain: “although these two compounds contain equally charged ions, sodium oxides have a shorter ionic distance thus the force of attraction is stronger”
- Process: when given lattice energy question, write out the charges above each ion (ignore subscripts); then, if needed, compare sizes of atoms
Partial Ionic Character of Covalent Bonds
- All bonds have a certain percent ionic character
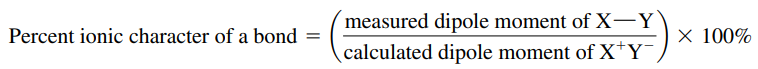
- Compounds with more than 50% ionic character are usually ionic
- Any compound that conducts an electric current when melted will be classified as ionic.
The Localized Electron Model
- Localized electron (LE) model: assumes that bonds and molecules are formed from the overlapping of atomic orbitals on diff. atoms
- Electron pairs are assumed to be localized on a particular atom or in the space between two atoms
- Lone pairs: belong to one atom/nuclei
- Bonding pairs: belong to 2 atoms/nuclei → found in the space between the atoms
- Delocalization: electrons are free to move throughout the entire molecule; can be with either atom