Lewis Diagrams Study Notes- AP Chemistry- New Syllabus 2024-2025
2.5 Lewis Diagrams Study Notes – AP Chemistry
Lewis Diagrams Study Notes – AP Chemistry as per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
- Represent a molecule with a Lewis diagram.
Key Concepts:
- Drawing Lewis Diagrams
Drawing Lewis Diagrams
A Lewis diagram (or Lewis structure) represents the arrangement of valence electrons in a molecule. It shows how atoms are bonded and where lone pairs of electrons are located.
Key Properties: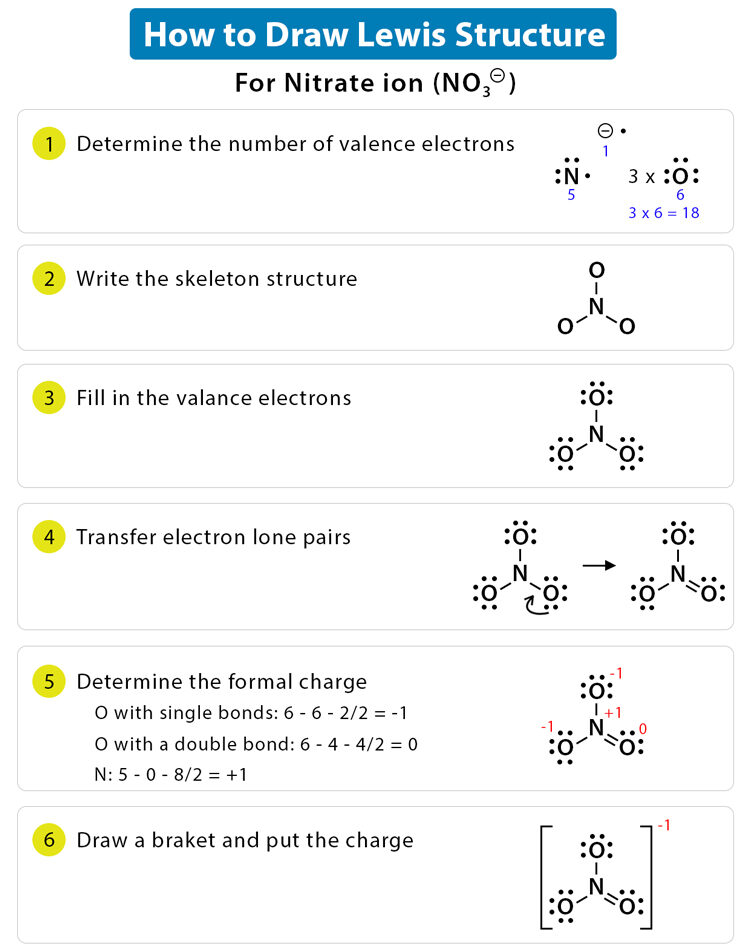
- Valence Electrons: Only valence electrons are represented (dots or lines).
- Bonds: Shared pairs of electrons (covalent bonds) are shown as lines between atoms.
- Lone Pairs: Nonbonding electron pairs are shown as dots around atoms.
- Octet Rule: Atoms (except H and He) tend to achieve 8 electrons in their valence shell.
Exceptions:
- Hydrogen: Stable with 2 electrons.
- Expanded Octets: Atoms in period 3 or higher (e.g., S, P, Xe) can hold more than 8 electrons.
- Electron-Deficient Atoms: Some atoms (e.g., B, Be) may have fewer than 8 electrons.
Steps for Drawing a Lewis Diagram:
- Count the total number of valence electrons in the molecule/ion.
- Arrange atoms (least electronegative atom in the center, hydrogen never central).
- Place single bonds between the central atom and outer atoms.
- Distribute remaining electrons to satisfy the octet rule.
- Form double or triple bonds if necessary to complete octets.
Key Idea: A Lewis diagram is a 2D representation that shows bonding and lone pairs, helping predict molecular structure, polarity, and reactivity.
Example :
Draw the Lewis diagram for \(\mathrm{CO_2}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: Carbon (4) + Oxygen (6 × 2) = 16 electrons.
Step 2: Place carbon in the center with two oxygen atoms bonded to it.
Step 3: Place single bonds first → 4 electrons used, 12 left.
Step 4: Distribute remaining electrons to complete octets: each oxygen gets 6 more electrons (12 total used).
Step 5: Carbon has only 4 electrons, so convert lone pairs into double bonds with each oxygen.
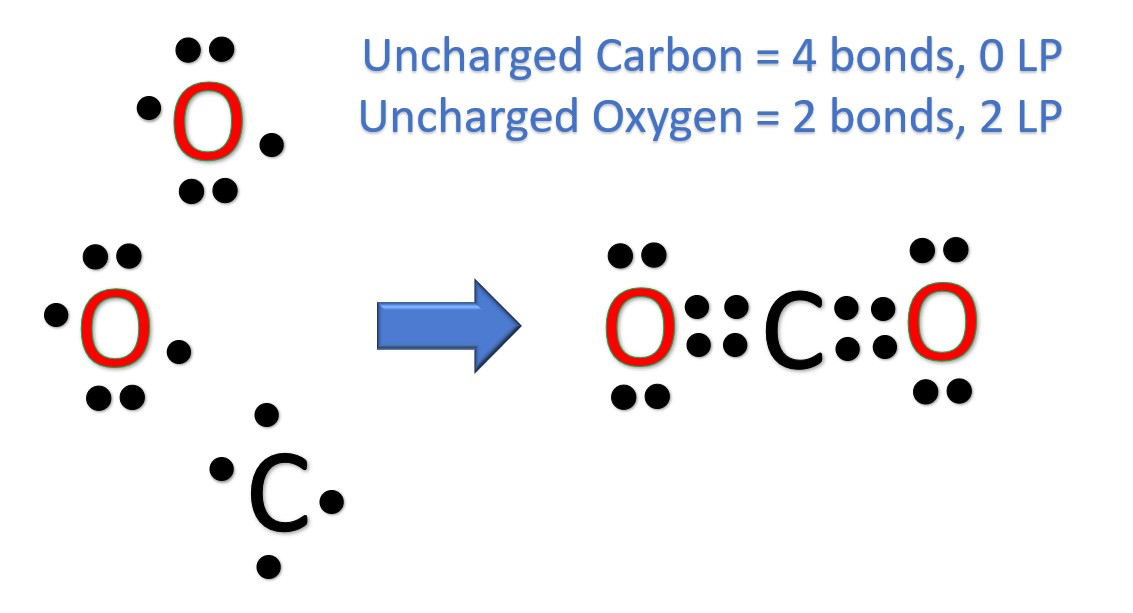
Final Answer: The Lewis diagram is:
O = C = O, with each oxygen having two lone pairs.
Example :
Draw the Lewis diagram for \(\mathrm{H_2O}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: H (1×2) + O (6) = 8 electrons.
Step 2: Place O in the center, bond two H atoms to O with single bonds (4 electrons used).
Step 3: 4 electrons remain → place them as 2 lone pairs on O.
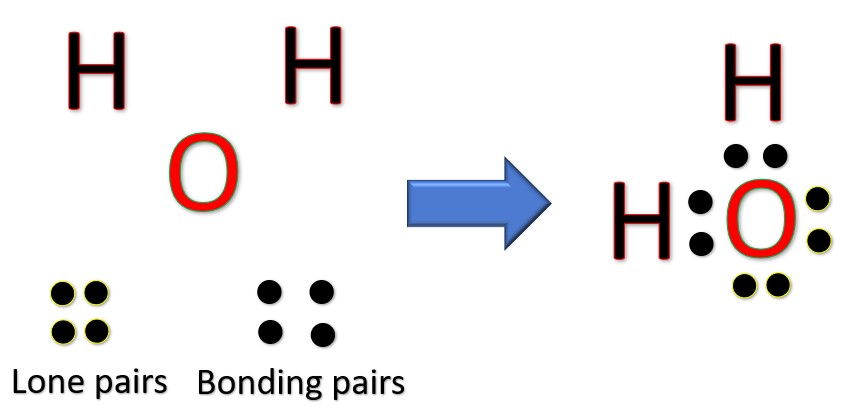
Final Answer: O has 2 single bonds and 2 lone pairs: H–O–H (with two lone pairs on O).
Example :
Draw the Lewis diagram for \(\mathrm{NH_3}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: N (5) + H (1×3) = 8 electrons.
Step 2: Place N in the center, bond three H atoms to N (6 electrons used).
Step 3: 2 electrons remain → lone pair on N.
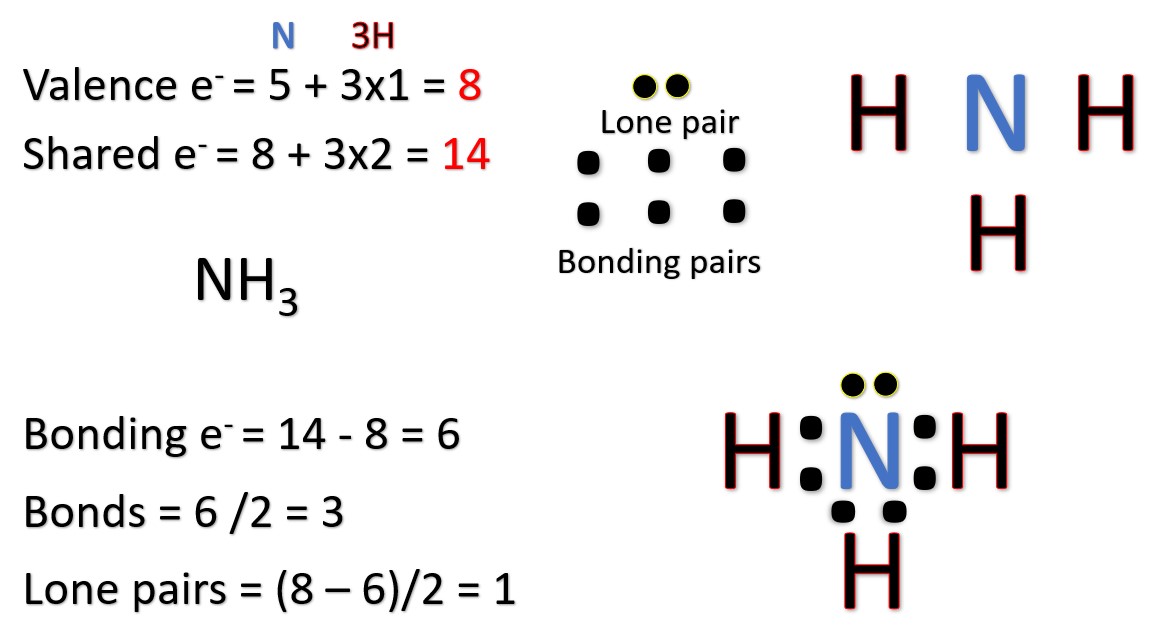
Final Answer: N has 3 single bonds and 1 lone pair: H | H–N–H (with a lone pair on N).
Example :
Draw the Lewis diagram for the polyatomic ion \(\mathrm{NO_3^-}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: N (5) + O (6×3) + 1 (extra for charge) = 24 electrons.
Step 2: Place N in the center, bond to 3 O atoms with single bonds (6 electrons used).
Step 3: 18 electrons left → distribute to O atoms to complete octets.
Step 4: N has only 6 electrons → make one double bond with an O.
Step 5: Place brackets with a –1 charge.
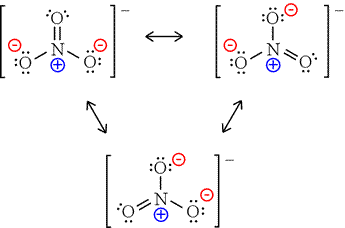
Final Answer: Resonance structures: one N=O double bond, two N–O single bonds, with negative charge delocalized. The actual structure is a resonance hybrid.
Example :
Draw the Lewis diagram for \(\mathrm{SO_4^{2-}}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: S (6) + O (6×4) + 2 (charge) = 32 electrons.
Step 2: Place S in the center, bond to 4 O atoms with single bonds (8 electrons used).
Step 3: 24 electrons remain → give 3 lone pairs to each O (24 used).
Step 4: S already has 8 electrons (octet satisfied), but expanded octet is possible since S is in Period 3.
Step 5: Can form resonance structures with S=O double bonds.
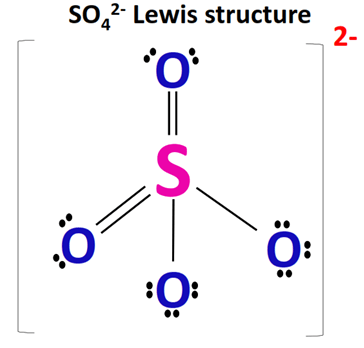
Final Answer: \(\mathrm{SO_4^{2-}}\) has resonance with two S=O double bonds and two S–O single bonds, all equivalent in the resonance hybrid. Place brackets with –2 charge.
