Resonance and Formal Charge- AP Chemistry Notes- New Syllabus 2024-2025
Resonance and Formal Charge Notes -AP Chemistry Note
Resonance and Formal Charge Notes -AP Chemistry Note – AP Chemistry as per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
- Represent a molecule with a Lewis diagram that accounts for resonance between equivalent structures or that uses formal charge to select between nonequivalent structures.
Key Concepts:
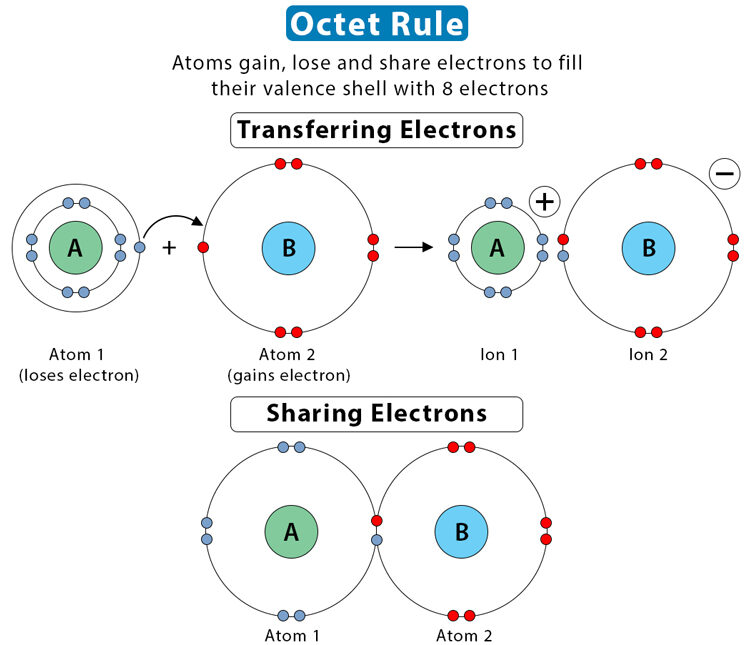
- Resonance in Lewis Structures
- Octet Rule and Formal Charge
- Limitations of the Lewis Structure Model
Resonance in Lewis Structures
Resonance occurs when more than one valid Lewis structure can be drawn for the same molecule or ion by shifting the positions of electrons (not atoms). The actual structure is a resonance hybrid a blend of all contributing structures.
Key Properties:
- Equivalent Lewis Structures: When two or more structures can be drawn with the same arrangement of atoms but different placements of electrons, resonance must be considered.
- Delocalization of Electrons: Resonance represents delocalized electrons spread across multiple atoms, not fixed in one bond.
- Stability: The resonance hybrid is more stable than any single contributing structure because electron density is distributed.
- Bond Properties: Resonance explains observed bond lengths that are intermediate between single and double bonds.
Key Idea: Resonance is required whenever more than one equivalent Lewis structure exists. It improves accuracy in predicting molecular structure and properties.
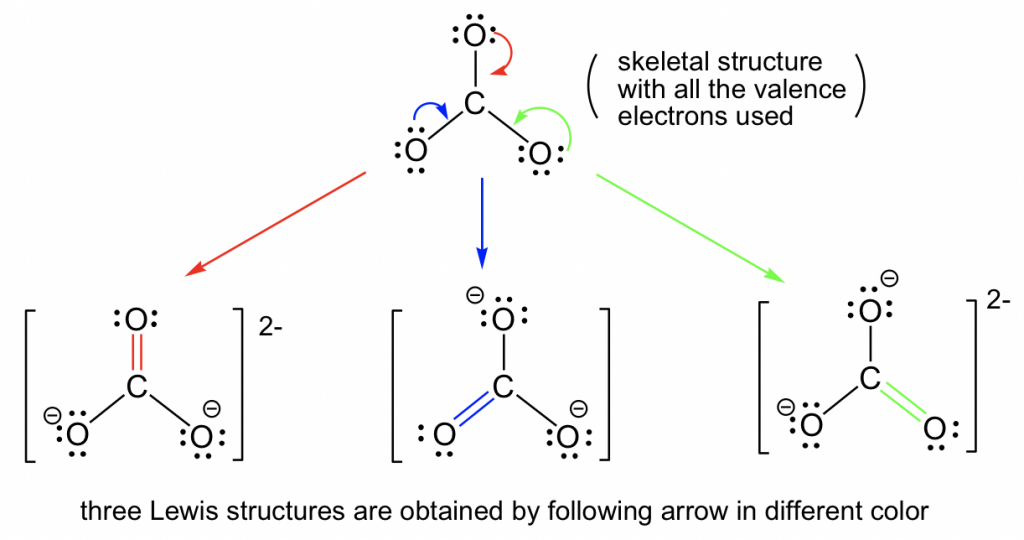
Example :
Explain why the bond lengths in the carbonate ion \(\mathrm{CO_3^{2-}}\) are all equal, even though a simple Lewis structure suggests otherwise.
▶️ Answer/Explanation
Step 1: A single Lewis structure for \(\mathrm{CO_3^{2-}}\) shows one C=O double bond and two C–O single bonds.
Step 2: However, equivalent structures can be drawn with the double bond in different locations.
Step 3: Resonance requires combining these equivalent structures into a hybrid.
Step 4: In the resonance hybrid, all three C–O bonds are identical, with bond order between 1 and 2.
Final Answer: The carbonate ion has delocalized electrons, making all three C–O bonds equal in length due to resonance.
Octet Rule and Formal Charge
When multiple valid Lewis structures are possible, the octet rule and formal charge analysis are used to determine which structure best represents the molecule. The most accurate Lewis structure minimizes formal charges and places negative charges on the most electronegative atoms.
Key Properties:
Octet Rule:
- Atoms (especially C, N, O, and F) tend to achieve 8 valence electrons.
- Structures that satisfy the octet rule are generally more stable
Formal Charge: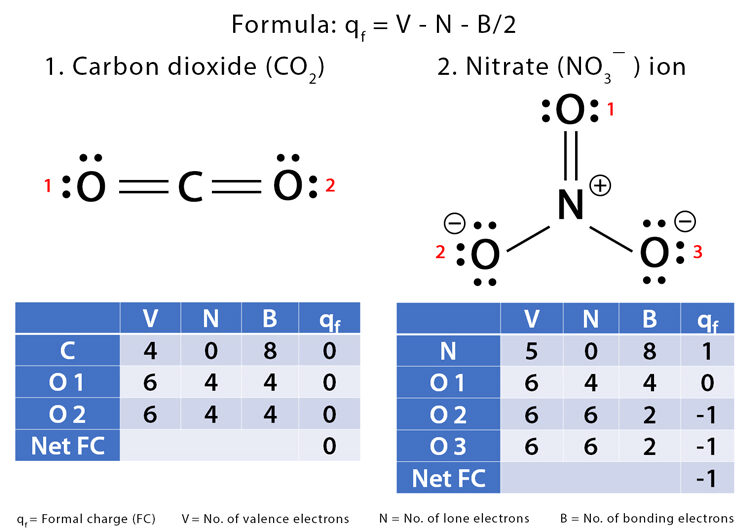
\(\mathrm{Formal\ Charge = Valence\ Electrons – (Nonbonding\ Electrons + \tfrac{1}{2}Bonding\ Electrons)}\)
- The best Lewis structure has the smallest possible formal charges.
If charges are present, negative formal charges should be on more electronegative atoms.
- Overall Charge: The sum of formal charges must equal the actual charge of the molecule or ion.
Key Idea: The best Lewis diagram is the one that minimizes formal charges and obeys the octet rule, providing the most accurate model for molecular structure and properties.
Example :
Which Lewis structure is more stable for the cyanate ion, \(\mathrm{OCN^-}\): one with a negative charge on oxygen or one with a negative charge on nitrogen?
▶️ Answer/Explanation
Step 1: Draw two possible Lewis structures: (a) O=C=N⁻ → negative charge on nitrogen. (b) ⁻O–C≡N → negative charge on oxygen.
Step 2: Apply formal charge calculation:
- In (a): Oxygen formal charge = 0, Nitrogen formal charge = –1.
- In (b): Oxygen formal charge = –1, Nitrogen formal charge = 0.
Step 3: Since oxygen is more electronegative than nitrogen, it is better suited to carry the negative charge.
Final Answer: Structure (b), ⁻O–C≡N, is the more stable Lewis structure because it minimizes formal charges and places the negative charge on the more electronegative atom.
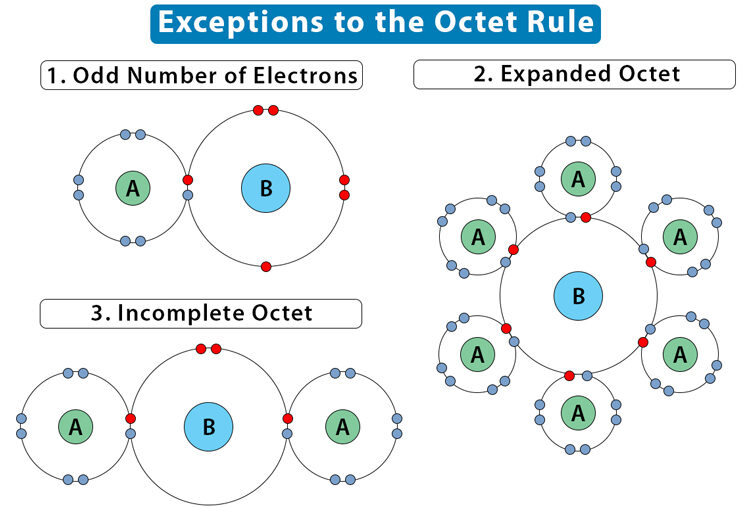
Limitations of the Lewis Structure Model
While Lewis structures are a powerful tool for representing covalent bonding, they have limitations. In particular, molecules or ions with an odd number of valence electrons, expanded octets, or delocalized bonding cannot always be represented accurately by a single Lewis diagram.
Key Properties:
Odd-Electron Species (Free Radicals):
- Some molecules, like \(\mathrm{NO}\) or \(\mathrm{ClO_2}\), have an odd number of electrons.
- Lewis structures cannot assign complete octets to all atoms in these species.
Expanded Octets:
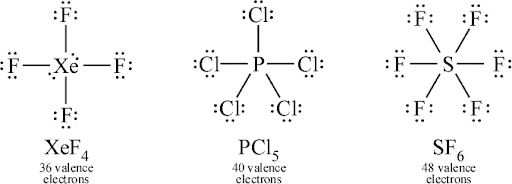
- Atoms in period 3 or beyond (e.g., \(\mathrm{PCl_5}\), \(\mathrm{SF_6}\)) may hold more than 8 valence electrons.
- Lewis diagrams must allow for expanded octets, which the simple octet rule does not predict.
Resonance and Delocalization:

- In cases like \(\mathrm{O_3}\) or \(\mathrm{CO_3^{2-}}\), electron density is delocalized.
- A single Lewis diagram cannot fully represent the real bonding situation.
Key Idea: Lewis structures are an essential model, but they are limited when dealing with odd-electron species, expanded octets, and delocalized resonance. More advanced models (like molecular orbital theory) are required for full accuracy.
Example :
Why can’t the Lewis structure of nitric oxide (\(\mathrm{NO}\)) satisfy the octet rule for both atoms?
▶️ Answer/Explanation
Step 1: Nitric oxide has 11 valence electrons (5 from N + 6 from O).
Step 2: When drawing a Lewis structure, 11 is an odd number, so at least one atom cannot achieve a complete octet.
Step 3: The best Lewis structure shows a double bond between N and O with one unpaired electron on nitrogen.
Final Answer: Because NO has an odd number of valence electrons, the octet rule cannot be satisfied for both atoms simultaneously. This highlights a limitation of the Lewis structure model.
Example :
Draw the Lewis diagram for \(\mathrm{BF_3}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: B (3) + F (7×3) = 24 electrons.
Step 2: Place B in the center, bond to 3 F atoms (6 electrons used).
Step 3: Distribute remaining 18 electrons as lone pairs on F atoms.
Step 4: B has only 6 electrons → does not complete octet.
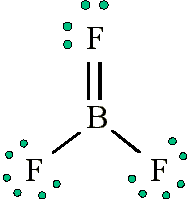
Final Answer: \(\mathrm{BF_3}\) is stable with an incomplete octet on B (exception).
Example :
Draw the Lewis diagram for \(\mathrm{SF_6}\).
▶️ Answer/Explanation
Step 1: Count valence electrons: S (6) + F (7×6) = 48 electrons.
Step 2: Place S in the center, bond to 6 F atoms with single bonds (12 electrons used).
Step 3: Distribute remaining 36 electrons as lone pairs on the F atoms (each F gets 3 pairs).
Step 4: S has 12 electrons (expanded octet, allowed since Period 3).
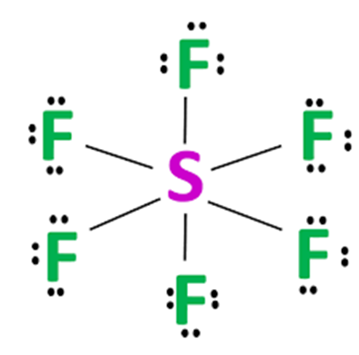
Final Answer: \(\mathrm{SF_6}\) is stable with an expanded octet on S (exception).