Resonance
- Resonance: is used when more than one valid Lewis structure can be written for a molecule (can move a double bond between the same elements)
- Actual electron structure of the molecule is an average of these resonance structures.
- Bond order:
- Single bond has BO of 1; Double bond has BO of 2 → length of single bond is longer
- Ex:
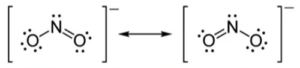
- Double bond isn’t either left or right, but rather split between two locations
- The bond order of N is 1.5 (3 bonds are split between 2 locations)
- Note: questions involving bond length will often involve resonance → have to draw out all resonance structures and use double arrows to indicate that the real molecule is an avg of all three
Formal Charge
- Formal charge can be used when there are diff lewis structures that can be drawn for a molecule
- Best Lewis structure will have formal charges closest to zero and with any (-) formal charge on most electronegative atom
- On AP exam will usually have 2 already drawn structures and ask which one is more valid
- Formal Charge = Valence Electrons – (Lone pairs [dots] + Bonded pairs [lines]
- What if they have the same number of zeroes?
- The “better” structure has the more negative charge on the more electronegative element
