Intermolecular Properties Notes - AP Chemistry -New Syllabus 2024-2025
AP Chemistry – Intermolecular Properties Notes
AP Chemistry – Intermolecular Properties Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the chemical structures of molecules and the relative strength of their intermolecular forces when:
- The molecules are of the same chemical species
- The molecules are of two different chemical species.
Key Concepts:
- London Dispersion Forces
- Dipole-Induced Dipole Interactions
- Dipole-Dipole Interactions
- Ion-Dipole Interactions
- Molecular Dipole Moment
- Hydrogen Bonding
- Interactions in Large Biomolecules
London Dispersion Forces
London dispersion forces are weak, temporary intermolecular attractions that arise due to momentary fluctuations in electron density within molecules or atoms. These fluctuations create temporary dipoles that induce dipoles in neighboring molecules, leading to Coulombic attraction between them.
Nature of Interaction: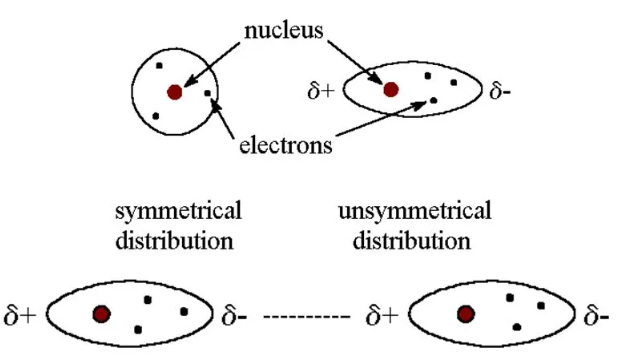
- Caused by instantaneous dipoles that appear and disappear as electrons move around the nucleus.
- Attractive forces exist between temporary dipoles and induced dipoles in adjacent molecules.
- These are universal forces — present in all atoms and molecules, polar or nonpolar.
Dependence on Contact Area:
- Dispersion forces increase with greater surface contact between molecules.
- Elongated or flat molecules (like \(\mathrm{C_5H_{12}}\) isomers) exhibit stronger LDFs than compact molecules.
Dependence on Polarizability:
- Polarizability = ease with which the electron cloud can be distorted to form a temporary dipole.
- Increases with:
- Larger number of electrons (heavier atoms or larger molecules).
- Greater electron cloud size (more diffuse valence electrons).
- Presence of π bonds (since π electrons are more easily distorted).
Relative Strength:
- Weakest of the intermolecular forces individually, but can be significant in large, heavy, or highly polarizable molecules.
- Often the dominant IMF in large nonpolar molecules like \(\mathrm{I_2}\), hydrocarbons, or noble gases at low temperatures.
Terminology:
- London dispersion forces are one type of van der Waals force.
- However, “van der Waals forces” is a broader term that includes both dispersion and dipole–dipole interactions — the two should not be used synonymously.
Example :
Explain why iodine (\(\mathrm{I_2}\)) is a solid at room temperature, while fluorine (\(\mathrm{F_2}\)) is a gas.
▶️ Answer/Explanation
Step 1: Both \(\mathrm{I_2}\) and \(\mathrm{F_2}\) are nonpolar molecules, so the only intermolecular forces present are London dispersion forces.
Step 2: Iodine has more electrons (Z = 53 per atom) and a larger, more polarizable electron cloud than fluorine (Z = 9).
Step 3: Greater polarizability means stronger temporary dipoles → stronger dispersion forces → higher boiling/melting points.
Final Answer: \(\mathrm{I_2}\) has stronger London dispersion forces due to higher polarizability, making it a solid, while \(\mathrm{F_2}\), with weak LDFs, is a gas at room temperature.
i. Dipole–Induced Dipole Interactions
A dipole–induced dipole interaction occurs when a polar molecule with a permanent dipole induces a temporary dipole in a nearby nonpolar molecule. The resulting attraction is always Coulombic and attractive.
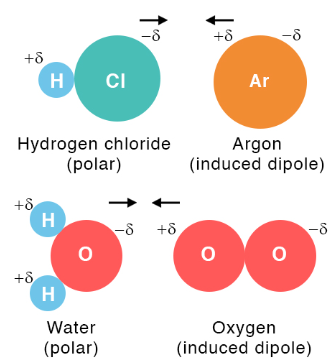
Key Properties:
- Occurs between a polar and a nonpolar molecule.
- The polar molecule’s electric field distorts the electron cloud of the nonpolar molecule, inducing a temporary dipole.
- The strength of the interaction depends on:
- The magnitude of the dipole moment of the polar molecule.
- The polarizability of the nonpolar molecule (how easily its electron cloud can be distorted).
- These forces are always attractive, though typically weaker than dipole–dipole or ion–dipole forces.
Key Idea: A polar molecule can temporarily polarize a nearby nonpolar molecule, leading to an attractive dipole-induced dipole interaction that strengthens with increasing polarizability.
Example :
Explain why oxygen (\(\mathrm{O_2}\)) is slightly soluble in water.
▶️ Answer/Explanation
Step 1: Water is a polar molecule with a permanent dipole; oxygen is nonpolar.
Step 2: The dipole of water induces a temporary dipole in \(\mathrm{O_2}\), creating a weak attraction.
Step 3: These dipole-induced dipole interactions allow limited solubility of \(\mathrm{O_2}\) in water.
Final Answer: Dipole–induced dipole interactions between water and oxygen molecules account for the slight solubility of oxygen in water.
ii. Dipole–Dipole Interactions
Dipole–dipole interactions occur between molecules that have permanent dipoles. The partially positive end of one molecule is attracted to the partially negative end of another.
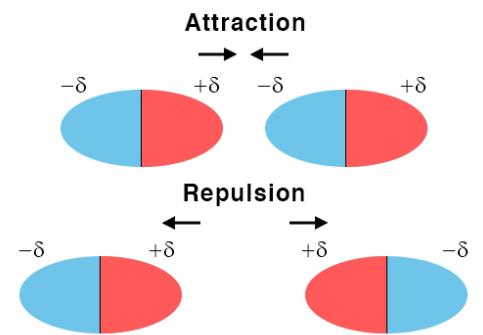
Key Properties:
- Present only between polar molecules.
- Strength depends on:
- The magnitude of eachdipolemoment (greater dipole = stronger attraction).
- The orientation of the dipoles — strongest when opposite ends align.
- Dipole–dipole interactions add to London dispersion forces, making polar molecules generally have higher boiling and melting points than nonpolar molecules of comparable size.
Key Idea: Polar molecules attract one another through dipole–dipole forces in addition to London dispersion forces, resulting in higher overall intermolecular attraction.
Example :
Compare the boiling points of \(\mathrm{CH_3Cl}\) and \(\mathrm{CH_4}\).
▶️ Answer/Explanation
Step 1: \(\mathrm{CH_3Cl}\) is polar; \(\mathrm{CH_4}\) is nonpolar.
Step 2: Both have London dispersion forces, but \(\mathrm{CH_3Cl}\) also has dipole–dipole interactions due to the polar C–Cl bond.
Step 3: Dipole–dipole interactions increase the total intermolecular forces in \(\mathrm{CH_3Cl}\).
Final Answer: \(\mathrm{CH_3Cl}\) has a higher boiling point than \(\mathrm{CH_4}\) because of additional dipole–dipole attractions between its polar molecules.
iii. Ion–Dipole Interactions
Ion–dipole forces occur between an ion and a polar molecule. These are among the strongest intermolecular forces, due to the full charge on the ion interacting with the partial charges on the polar molecule.
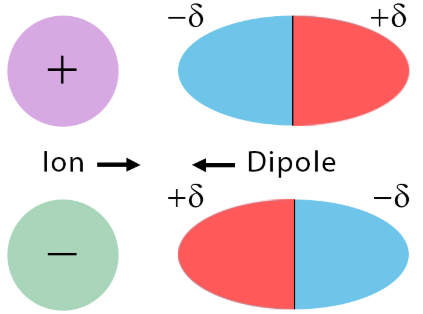
Key Properties:
- Common in solutions of ionic compounds in polar solvents (e.g., salt in water).
- Strength depends on:
- The charge and size of the ion (higher charge or smaller radius → stronger attraction).
- The dipole moment of the polar molecule.
- These interactions are stronger than dipole–dipole and London dispersion forces because they involve a full ionic charge.
Key Idea: Ion–dipole forces are the dominant attractive forces in ionic solutions, responsible for processes like dissolving salts in water.
Example :
Describe the interaction between \(\mathrm{Na^+}\) ions and water molecules in aqueous \(\mathrm{NaCl}\).
▶️ Answer/Explanation
Step 1: The \(\mathrm{Na^+}\) ion is positively charged, and water is a polar molecule with the oxygen end partially negative.
Step 2: The oxygen atoms in nearby water molecules orient toward the \(\mathrm{Na^+}\) ion.
Step 3: Strong ion–dipole attractions form between the ion and surrounding water dipoles, stabilizing the ion in solution.
Final Answer: Ion–dipole interactions between \(\mathrm{Na^+}\) and the oxygen atoms of water molecules allow ionic compounds like \(\mathrm{NaCl}\) to dissolve in polar solvents.
Relative Strength and Orientation Dependence of Dipole–Dipole and Ion–Dipole Forces
Both dipole–dipole and ion–dipole forces arise from electrostatic (Coulombic) interactions between charged or partially charged regions of molecules or ions. The strength and effectiveness of these forces depend on the magnitude of the charges involved and the spatial orientation of the interacting particles.
1. Nature of the Interactions:
Dipole–Dipole: Interaction occurs between two polar molecules. The positive end (\(\delta^+\)) of one molecule is attracted to the negative end (\(\delta^-\)) of another.
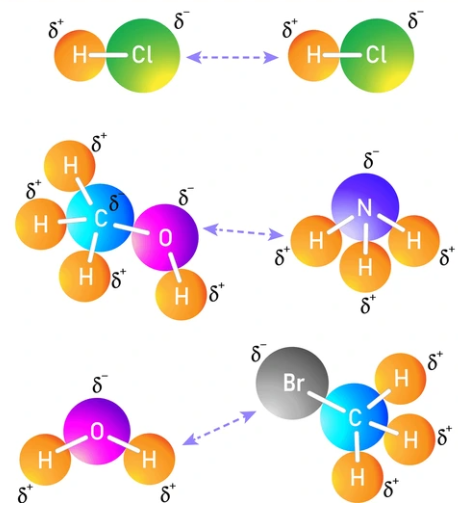
Ion–Dipole: Interaction occurs between an ion (full charge) and a polar molecule (partial charge). The ion’s electric field aligns the dipoles of surrounding molecules, forming strong directional attractions.
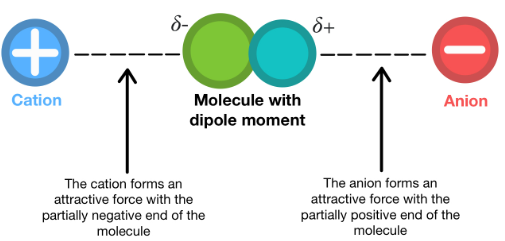
2. Strength Comparison:
- Ion–dipole forces are significantly stronger than dipole–dipole forces because they involve a full ionic charge rather than partial charges.
- Dipole–dipole forces are stronger than dispersion forces but weaker than ion–dipole forces.
- The overall strength follows this order:
Ion–Dipole > Dipole–Dipole > Dipole–Induced Dipole > London Dispersion
3. Orientation Dependence:
- These forces are highly directional — their strength depends on the relative alignment of dipoles or of the dipole with the ion’s electric field.
- Optimal orientation: When opposite charges (δ⁺ and δ⁻) are aligned along the same axis, attractive interactions are strongest.
- Non-optimal orientation: If dipoles are misaligned or like charges face each other, repulsion occurs, reducing the net attraction.
- At higher temperatures, random molecular motion disrupts alignment, decreasing the average dipole–dipole attraction.
4. Dependence on Partial Charges:
- The larger the dipole moment (difference in electronegativity and bond polarity), the stronger the dipole–dipole or ion–dipole attraction.
- In ion–dipole forces, smaller ions with higher charge density (like \(\mathrm{Na^+}\) or \(\mathrm{Mg^{2+}}\)) create stronger fields that better align nearby dipoles.
Example :
Explain why ion–dipole forces between \(\mathrm{Na^+}\) and water are stronger than dipole–dipole forces between water molecules.
▶️ Answer/Explanation
Step 1: Ion–dipole forces involve a full charge (\(\mathrm{Na^+}\)) and partial charges on the water molecule, while dipole–dipole forces involve only partial charges.
Step 2: The electric field of the \(\mathrm{Na^+}\) ion is much stronger, attracting the δ⁻ oxygen ends of nearby water molecules with greater force.
Step 3: The ion’s strong field also orients the dipoles perfectly, maximizing Coulombic attraction.
Final Answer: Ion–dipole interactions are stronger than dipole–dipole forces because they involve full ionic charges and optimal orientation of polar molecules around the ion.
Hydrogen Bonding
Hydrogen bonding is a particularly strong type of dipole–dipole interaction that occurs when a hydrogen atom, covalently bonded to a highly electronegative atom (N, O, or F), is electrostatically attracted to the lone pair of electrons on another electronegative atom (N, O, or F) in a different molecule or within a different part of the same molecule.
1. Origin of Hydrogen Bonding:
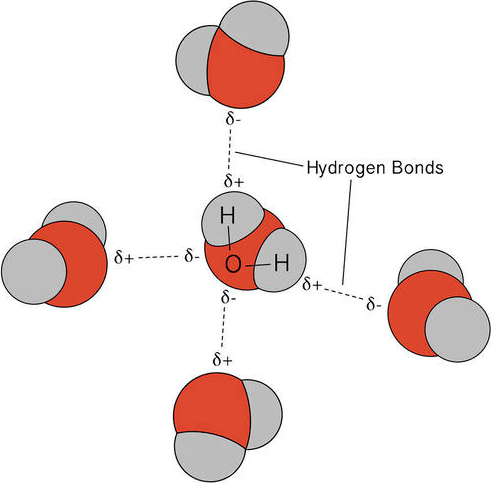
- Hydrogen has only one electron; when bonded to a highly electronegative atom (N, O, or F), the bonding electrons are drawn away, leaving the hydrogen nucleus (a bare proton) partially exposed.
- This exposed δ⁺ hydrogen strongly attracts the lone pair electrons on another nearby electronegative atom (δ⁻ region).
- This interaction forms a hydrogen bond, which is a noncovalent, electrostatic attraction stronger than typical dipole–dipole forces.
2. Conditions for Hydrogen Bonding: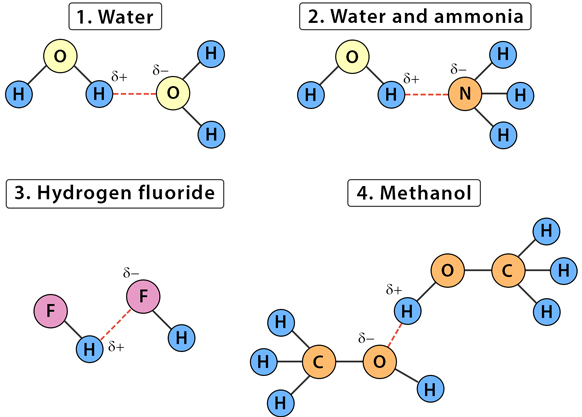
- Hydrogen must be covalently bonded to N, O, or F (most electronegative elements).
- There must be a lone pair of electrons on a neighboring N, O, or F atom to attract the hydrogen.
- Examples include: \(\mathrm{H_2O}\), \(\mathrm{NH_3}\), and \(\mathrm{HF}\).
3. Relative Strength:
- Hydrogen bonds are stronger than dipole–dipole or dispersion forces but weaker than covalent or ionic bonds.
- Typical hydrogen bond energies: ~10–40 kJ/mol (compared to ~1–10 kJ/mol for dispersion and ~400 kJ/mol for covalent bonds).
4. Orientation and Directionality: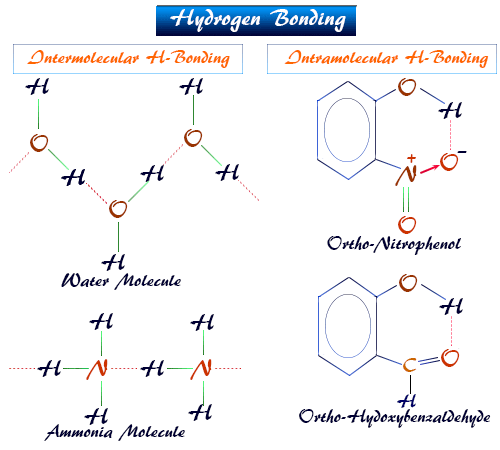
- Hydrogen bonds are highly directional — the strongest attraction occurs when the donor H–X bond and the acceptor atom’s lone pair align linearly.
- This directionality plays an important role in the structure of biological macromolecules such as DNA and proteins.
5. Intramolecular vs. Intermolecular Hydrogen Bonding:
- Intermolecular: Between molecules (e.g., between water molecules in liquid water).
- Intramolecular: Within a single molecule between two parts (e.g., in o-nitrophenol, where an –OH hydrogen bonds to a nearby oxygen atom).
6. Physical Consequences:
- Substances with hydrogen bonding have higher boiling and melting points compared to molecules of similar size without hydrogen bonds.
- Hydrogen bonding explains unique properties of water — high surface tension, high heat capacity, and solid water (ice) being less dense than liquid water.
Example :
Explain why water (\(\mathrm{H_2O}\)) has a much higher boiling point than hydrogen sulfide (\(\mathrm{H_2S}\)).
▶️ Answer/Explanation
Step 1: Both are molecular compounds with similar structures, but the key difference lies in the bonding element (O vs. S).
Step 2: Oxygen is much more electronegative than sulfur, allowing water molecules to form strong hydrogen bonds.
Step 3: In hydrogen sulfide, sulfur’s lower electronegativity prevents the formation of hydrogen bonds; only weak London dispersion forces are present.
Final Answer: The extensive hydrogen bonding network in \(\mathrm{H_2O}\) leads to a higher boiling point compared to \(\mathrm{H_2S}\), where such bonding does not occur.
Noncovalent Interactions in Large Biomolecules
In large biomolecules such as proteins, nucleic acids, and polysaccharides, noncovalent interactions occur either between separate molecules or within different regions of the same molecule. These interactions determine the molecule’s three-dimensional structure, chemical behavior, and biological function.
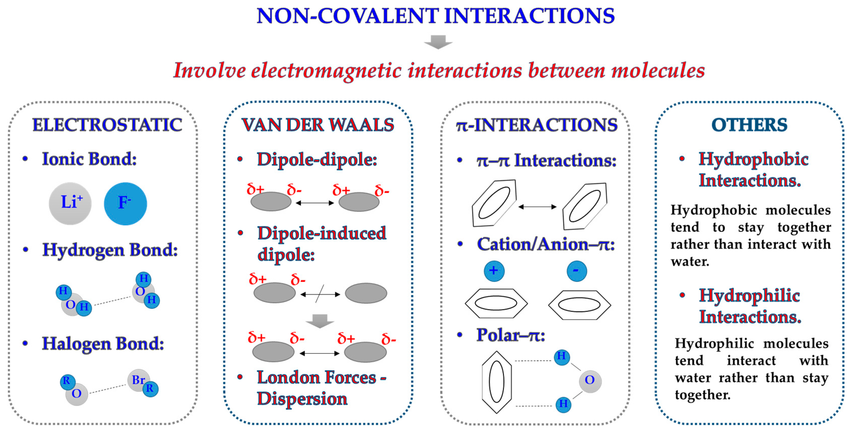
1. Nature of Noncovalent Interactions:
- Noncovalent forces are weaker than covalent bonds but are essential for maintaining the structural stability and flexibility of biomolecules.
- They are reversible and allow for molecular recognition and dynamic biological processes.
- Although individually weak, many such interactions act cooperatively to stabilize large macromolecular structures.
2. Major Types of Noncovalent Interactions:
- Hydrogen Bonding:Occurs between hydrogen atoms bonded to N, O, or F and the lone pairs on nearby electronegative atoms. Example: Base pairing in DNA — A–T and G–C pairs are stabilized by hydrogen bonds.
- Ionic or Ion–Dipole Interactions:Occur between oppositely charged side chains or polar regions of biomolecules. Example: Attraction between a lysine \( \mathrm{–NH_3^+} \) group and an aspartate \( \mathrm{–COO^-} \) group in a protein.
- Dipole–Dipole Interactions:Between polar regions of a molecule (e.g., peptide carbonyl and amide groups) that help stabilize folded configurations.
- London Dispersion Forces:Weak, temporary attractions between nonpolar regions; significant in densely packed hydrophobic cores. Example: Stabilization of nonpolar amino acid side chains within proteins.
- Hydrophobic Interactions:Nonpolar groups cluster away from water, minimizing contact with polar solvents and increasing system stability. Example: Folding of proteins and formation of lipid bilayers in membranes.
3. Intermolecular vs. Intramolecular Interactions:
- Intermolecular:Between distinct biomolecules (e.g., enzyme–substrate binding or DNA strand pairing).
- Intramolecular:Within different parts of the same molecule (e.g., folding of a single protein chain into its tertiary structure).
4. Biological Significance:
- Noncovalent forces collectively stabilize complex molecular shapes and enable reversible interactions necessary for life processes.
- They determine properties such as solubility, folding, and recognition between biomolecules.
- The flexibility of noncovalent interactions allows macromolecules to change conformation and adapt to environmental conditions.
Example :
Explain how noncovalent interactions maintain the tertiary structure of a protein.
▶️ Answer/Explanation
Step 1: Hydrogen bonds form between polar side chains and backbone groups, stabilizing α-helices and β-sheets.
Step 2: Nonpolar (hydrophobic) side chains aggregate inside the protein, driven by hydrophobic interactions and dispersion forces.
Step 3: Ionic attractions between oppositely charged side chains further stabilize the folded structure.
Final Answer: Multiple noncovalent forces—hydrogen bonding, ionic interactions, and hydrophobic effects—cooperate to fold and stabilize the protein’s functional 3D structure.
