Intramolecular vs Intermolecular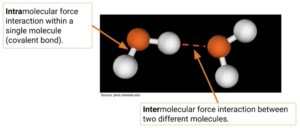
- Intramolecular bonding: what holds atoms together within the molecule
- Covalent and ionic
- Intermolecular forces: forces between (rather than within) molecules
Intermolecular Forces
- Weaker interactions that occur between molecules (3 types)
- Hydrogen bonding (strongest)
- Dipole-dipole forces (intermediate)
- London-dispersion forces (weakest)
- Intramolecular forces are a lot stronger than intermolecular forces
- The weaker the forces, the more likely the substance is to exist as a gas → bcuz particles are able to move far apart since they are not held together very strongly
- Molecules orient themselves to maximize the ⊕,⊝ interactions and to minimize ⊕,⊕ and ⊝,⊝ interactions
Dipole-Dipole Forces
- Dipole-Dipole Attraction: Molecules with dipole moments (polar molecules) can attract each other electrostatically by lining up so that the positive and negative ends are close to each other
- Can be attractive or repulsive
- The strength of the interactions is directly related to the magnitude of the dipole
- The more polar the molecule is (bigger electronegativity difference) → stronger the D-D attraction
- Force becomes weaker as the distance between the dipoles increases (larger atoms)
- Molecules with D-D have atoms held more tightly together → higher MP and BP → more likely to be a liquid at room temp
- The more polar the molecule is (bigger electronegativity difference) → stronger the D-D attraction
Hydrogen “Bonding”
- Hydrogen bonds: A very strong type of dipole-dipole attraction that occurs when hydrogen is covalently bonded (intramolecular) to N, O, or F
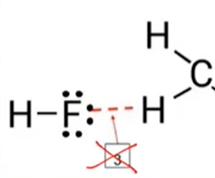
- → not HB bcuz H is covalently bonded to Carbon
- It is very strong due to the large difference in electronegativity and hydrogen’s small size that allows dipoles to be close → need more energy to overcome bonds → higher MP and BP
London Dispersion Forces
- ALL covalent compounds experience LDF (aka van der waal forces)
- Do not use the word “has”
- Is the only force present in noble gas atoms and nonpolar molecules
- Forces also exist between polar molecules but not as strong as D-D
- In non-polar molecules the electron charge is usually evenly distributed but it is possible that at a particular moment in time, the electrons might not be evenly distributed (e- are always moving in their orbitals) → distorts electron cloud → separation of charge creates instantaneous dipoles in molecule → induces another instantaneous dipole in another molecule → short/weak attraction between molecules
- Polarizability: how easy the electron cloud of an atom can be induced into a dipole
- Elements with more electrons (greater molar mass) = more polarizable molecule = electron cloud can be more easily induced into a dipole = stronger LDF = higher MP/BP
- Force is usually weak but can be significant in large atoms/molecules (Ex: F2, Cl2, Br2)
Ion-Dipole Forces
- Attractive force between an ion and the oppositely charged end of a polar molecule → strongest force
- Ex: NaCl and water
Forces Between Polar and Nonpolar Molecules
- Dipole-induced dipole interaction: a permanent dipole on a polar molecule can induce a dipole on a neighboring nonpolar molecule
- Thus polar molecules and nonpolar molecules exhibit an attraction for one another
- Explains why oxygen and CO2 can be dissolved (slightly) in water
- Thus polar molecules and nonpolar molecules exhibit an attraction for one another
- Factors that affect strength → nature of both molecules
- The larger the magnitude of the dipole in a polar molecule → the better able it is to induce a dipole in a neighboring molecule.
- Nonpolar molecules with a greater number of electrons have an increased polarizability (LDF) → dipole more easily induced
Factors Affected by IMF’s
Properties that increase with stronger InterMF’s → bcuz molecules experience stronger attractions for each other and it takes more energy to overcome that attraction
- Melting/Freezing and boiling points
- Surface tension
- Heat of vaporization
- Viscosity
Properties that decrease with stronger InterMF’s
- Vapor pressure
- Volatility: how easily a liquid evaporates
Comparing IMF’s
- If the size of molecules is similar → H-bonding > D-D > LDF
- If sizes are comparable → LDFs are similar bcuz have electron clouds of similar size and polarizability
- If the molecules are of different size → more complex → very imp. for AP exam
- LDF’s can become more significant than D-D or H-B when the size of molecules is bigger
- Occurs when the difference in magnitude of LDFs in A exceed the magnitude of all the other interactions in B, giving A more IMFs total
- LDF’s can become more significant than D-D or H-B when the size of molecules is bigger
- More linear compounds (less branched) = greater surface area for contact between molecules = stronger InterMFs
- Ex: for questions involving isomers
