AP Chemistry Unit 3.2 Properties of Solids Notes -New Syllabus 2024-2025
AP Chemistry Unit 3.2 Properties of Solids Notes
AP Chemistry Unit 3.2 Properties of Solids Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship among the macroscopic properties of a substance, the particulate-level structure of the substance, and the interactions between these particles.
Key Concepts:
- Relationship Between Intermolecular Forces and Physical Properties
- Particulate-Level Representations and Intermolecular Interactions
- Structure and Properties of Ionic Solids
- Covalent Network Solids
- Metallic Solids
- Metallic Solids and Alloys
Noncovalent Interactions in Large Biomolecules and Polymers
Relationship Between Intermolecular Forces and Physical Properties
The physical properties of liquids and solids—such as boiling point, melting point, vapor pressure, and volatility—are determined by the strength and type of intermolecular forces (IMFs) present between particles. Stronger intermolecular attractions require more energy to separate molecules, influencing a substance’s phase behavior and temperature-dependent properties.
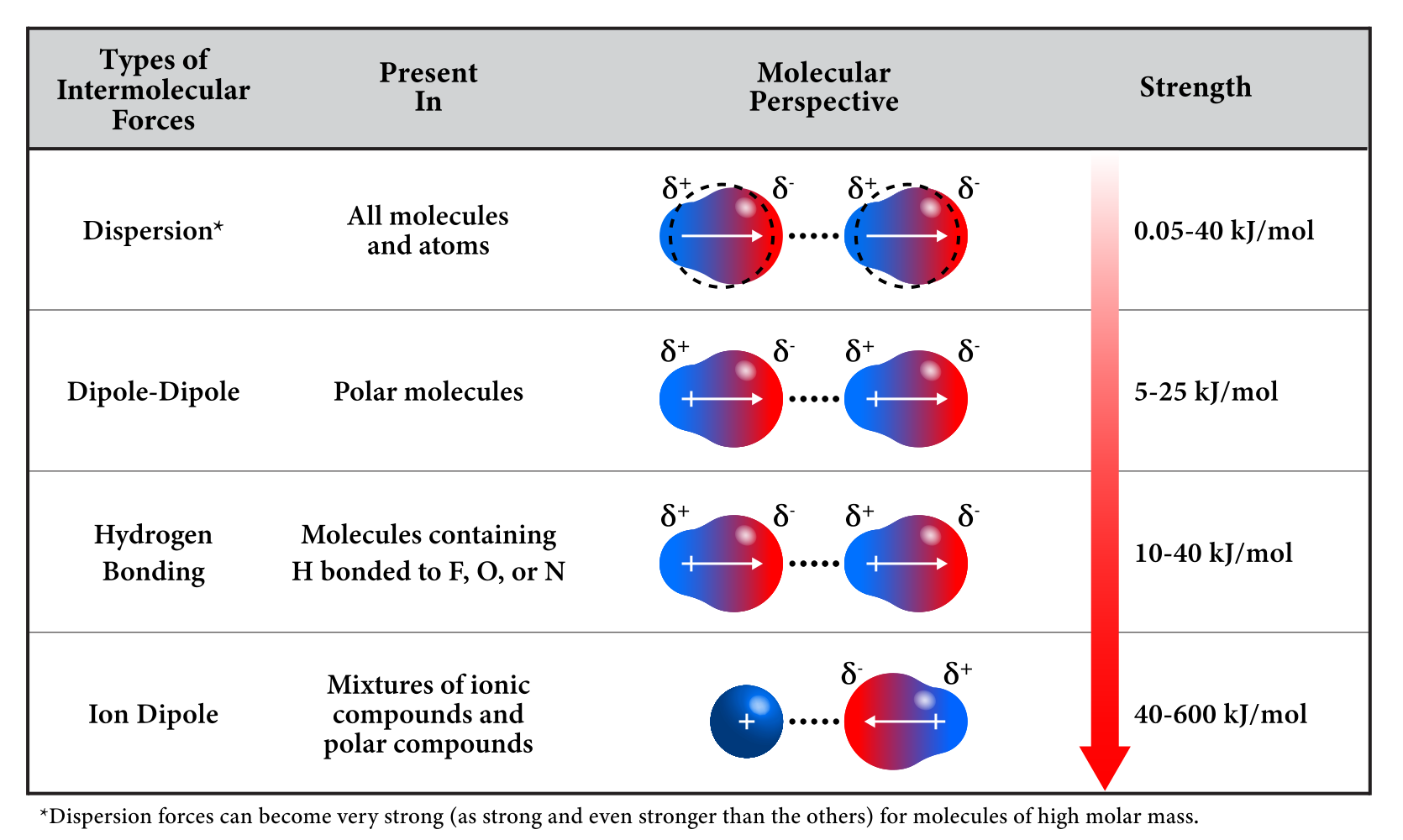
1. Intermolecular Forces and Phase Behavior:
- In the liquid and solid states, molecules are held together by intermolecular forces.
- When a substance vaporizes or melts, these interactions must be overcome (partially or completely).
- The stronger the IMFs, the more energy (in the form of heat) is required to separate molecules → higher phase change temperatures.
2. Boiling Point and Intermolecular Forces:
- The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure.
- Stronger IMFs mean molecules require more energy to escape the liquid phase → higher boiling point.
- Example: \(\mathrm{H_2O}\) (hydrogen bonding) boils at 100°C, while \(\mathrm{H_2S}\) (dipole–dipole and dispersion) boils at –60°C.
3. Vapor Pressure and IMF Strength:
- Vapor pressure is the pressure exerted by a vapor in equilibrium with its liquid at a given temperature.
- Substances with weaker IMFs allow molecules to escape more easily → higher vapor pressure.
- Substances with stronger IMFs have lower vapor pressure because fewer molecules can overcome the attraction to enter the gas phase.
- Inverse Relationship: Stronger IMFs → Lower Vapor Pressure → Higher Boiling Point.
4. Melting Point and IMF Strength:
- When a solid melts, intermolecular forces are not completely broken but rearranged.
- Stronger forces lead to higher melting points, though the trend is less direct than for boiling points because solids vary in structure (e.g., molecular, ionic, metallic, covalent network).
- Example: Ionic solids like \(\mathrm{NaCl}\) have higher melting points than molecular solids like \(\mathrm{I_2}\) because ionic bonds are much stronger than dispersion forces.
5. Types of Intermolecular Forces and Their Relative Strength:
| Type of Force | Relative Strength | Example | Effect on Boiling Point |
|---|---|---|---|
| London Dispersion | Weakest | \(\mathrm{CH_4}\), \(\mathrm{I_2}\) | Low |
| Dipole–Dipole | Moderate | \(\mathrm{CH_3Cl}\), \(\mathrm{HCl}\) | Medium |
| Hydrogen Bonding | Strong | \(\mathrm{H_2O}\), \(\mathrm{NH_3}\), \(\mathrm{HF}\) | High |
| Ion–Dipole | Very Strong | \(\mathrm{Na^+}\) in \(\mathrm{H_2O}\) | Very High |
Key Idea: The strength and type of intermolecular forces determine a substance’s physical properties. Stronger intermolecular attractions result in higher boiling and melting points, lower vapor pressures, and lower volatility.
Example :
Explain why ethanol (\(\mathrm{CH_3CH_2OH}\)) has a higher boiling point than dimethyl ether (\(\mathrm{CH_3OCH_3}\)), even though both have the same molecular formula (\(\mathrm{C_2H_6O}\)).
▶️ Answer/Explanation
Step 1: Both compounds have similar molar masses and dispersion forces.
Step 2: Ethanol can form hydrogen bonds (–OH group), while dimethyl ether cannot (no H bonded to O).
Step 3: Hydrogen bonding provides stronger intermolecular attraction, requiring more energy to separate molecules.
Final Answer: Ethanol’s hydrogen bonding increases its boiling point compared to dimethyl ether, which relies only on dipole–dipole and dispersion forces.
Particulate-Level Representations and Intermolecular Interactions
Particulate-level representations are visual models that depict individual atoms, ions, or molecules and the interactions among them. These diagrams help explain how microscopic interactions give rise to the observable, macroscopic properties of substances.
1. Purpose of Particulate Representations: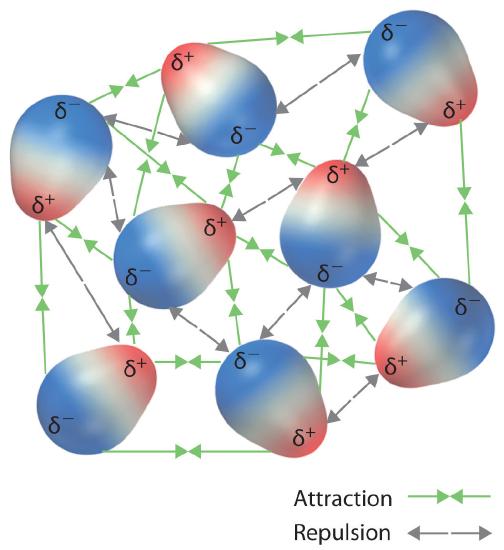
- They show how particles (atoms, ions, or molecules) interact through electrostatic forces and intermolecular attractions.
- They help connect molecular-level structure to bulk properties such as phase (solid, liquid, gas), solubility, melting/boiling point, and conductivity.
2. Features of Effective Particulate Diagrams:
- Display the correct relative sizes and charges of ions or atoms.
- Show directional interactions (e.g., hydrogen bonding, ionic attraction, or dipole alignment).
- Illustrate how increasing or decreasing interaction strength affects spacing and motion between particles.
3. Linking Microscopic Interactions to Macroscopic Properties:
- Strong intermolecular or ionic interactions → low vapor pressure, high boiling/melting point.
- Weak intermolecular interactions → high volatility, low melting and boiling points.
- In solids, the arrangement and strength of forces between particles determine hardness, brittleness, and conductivity.
Example :
Describe how a particulate diagram could represent water’s high boiling point compared to methane (\(\mathrm{CH_4}\)).
▶️ Answer/Explanation
Step 1: The water diagram would show molecules connected by directional hydrogen bonds (O–H···O) forming a network.
Step 2: The methane diagram would show weak London dispersion interactions with no hydrogen bonding.
Step 3: Strong hydrogen bonding in water explains its higher boiling point compared to methane, which lacks such forces.
Final Answer: The particulate diagram of water illustrates strong, directional hydrogen bonding responsible for its macroscopic property—high boiling point.
Structure and Properties of Ionic Solids
Ionic solids are composed of repeating patterns of positively and negatively charged ions held together by strong electrostatic (Coulombic) forces. The microscopic arrangement of ions explains their characteristic physical properties.
1. Ionic Bonding and Structure: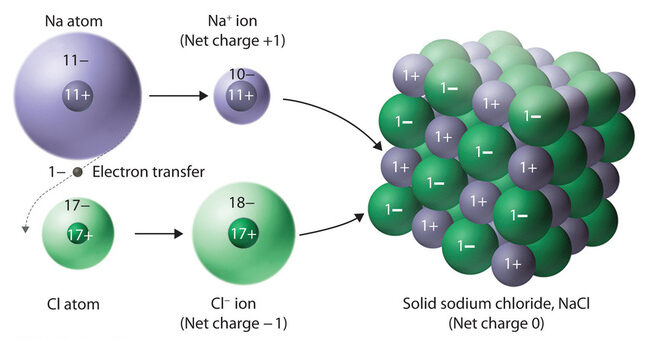
- Ions are arranged in a crystalline lattice to maximize attractions between opposite charges and minimize repulsions between like charges.
- The lattice structure is rigid and highly ordered, leading to strong ionic interactions throughout the solid.
2. Strength and Energy:
- Electrostatic attraction between ions follows Coulomb’s Law: \( \mathrm{F \propto \dfrac{q_1 q_2}{r^2}} \)
- Higher ionic charges and smaller ionic radii increase lattice energy, resulting in stronger bonding and higher melting points.
3. Macroscopic Properties of Ionic Solids:
- Low Vapor Pressure:Ionic solids have very strong interactions between ions; very few particles escape to the gas phase.
- High Melting and Boiling Points:Large amounts of energy are needed to overcome strong Coulombic attractions.
- Brittleness:When layers of ions are forced to shift, like charges align and repel, causing the crystal to fracture.
- Electrical Conductivity:Ionic solids do not conduct electricity in solid form (ions are fixed in place). They conduct electricity when melted or dissolved in water, because ions are mobile and can carry charge.
4. Example Correlation:
- \(\mathrm{NaCl}\): high melting point (~801°C), non-conductive as a solid, but conductive in molten or aqueous states.
- \(\mathrm{MgO}\): even higher melting point (~2850°C) due to higher charges (+2/–2) and smaller ionic radii → stronger lattice forces.
Example :
Explain why solid \(\mathrm{NaCl}\) does not conduct electricity, but molten \(\mathrm{NaCl}\) does.
▶️ Answer/Explanation
Step 1: In the solid state, \(\mathrm{Na^+}\) and \(\mathrm{Cl^-}\) ions are locked in fixed positions within the lattice and cannot move.
Step 2: In the molten state, the lattice breaks down, allowing ions to move freely and carry electric current.
Final Answer: Solid \(\mathrm{NaCl}\) is an insulator, while molten \(\mathrm{NaCl}\) conducts electricity because of mobile ions.
Covalent Network Solids
Covalent network solids are solids in which atoms are bonded together through an extensive network of strong covalent bonds. These structures extend in either three dimensions (e.g., diamond, silicon dioxide) or in two-dimensional layers (e.g., graphite). They are composed entirely of nonmetals or metalloids.
![]()
1. Composition and Structure:
- Formed from elements with strong directional covalent bonds, such as C, Si, and SiO₂.

- Can exist as:
- 3D networks — atoms bonded in a rigid lattice (e.g., diamond, SiC, SiO₂).
- 2D networks — layers of covalently bonded sheets with weak interlayer forces (e.g., graphite).
2. Bonding Characteristics:
- Each atom is covalently bonded to several others with fixed bond angles → high rigidity and structural stability.
- Breaking the solid requires breaking covalent bonds → very high melting points and hardness.
3. Physical Properties:![]()
- High Melting Point:Covalent bonds throughout the structure require large energy input to break.
- Hardness and Rigidity:Fixed bond angles and continuous bonding create very hard solids (e.g., diamond).
- Poor Conductivity:Electrons are localized in σ and π bonds, not free to move (except in graphite, where delocalized electrons allow conductivity).
- Variable Texture:Diamond is extremely hard, while graphite is soft and slippery due to weak forces between layers.
Examples and Bonding Summary:
| Substance | Type of Bonding | Structure | Properties |
|---|---|---|---|
| Diamond (C) | C–C covalent bonds | 3D tetrahedral network | Extremely hard, nonconductive, high melting point |
| Graphite (C) | C–C covalent in layers, weak dispersion between layers | 2D sheets of hexagonal layers | Soft, conductive (due to delocalized π electrons) |
| Silicon Dioxide (SiO₂) | Si–O covalent bonds | 3D tetrahedral network | Hard, brittle, nonconductive, very high melting point |
Example :
Explain why diamond and graphite, both forms of carbon, have different physical properties.
▶️ Answer/Explanation
Step 1: In diamond, each carbon forms four covalent bonds in a 3D tetrahedral network — rigid and strong.
Step 2: In graphite, each carbon forms three bonds, creating 2D layers with delocalized electrons and weak interlayer forces.
Final Answer: Diamond is hard and nonconductive due to 3D bonding; graphite is soft and conductive due to 2D sheets and delocalized π electrons.
Molecular Solids
Molecular solids consist of discrete, covalently bonded molecules held together in the solid by relatively weak intermolecular forces (IMFs), such as London dispersion, dipole–dipole, or hydrogen bonding. Because these interactions are weak compared to covalent or ionic bonds, molecular solids typically have low melting and boiling points.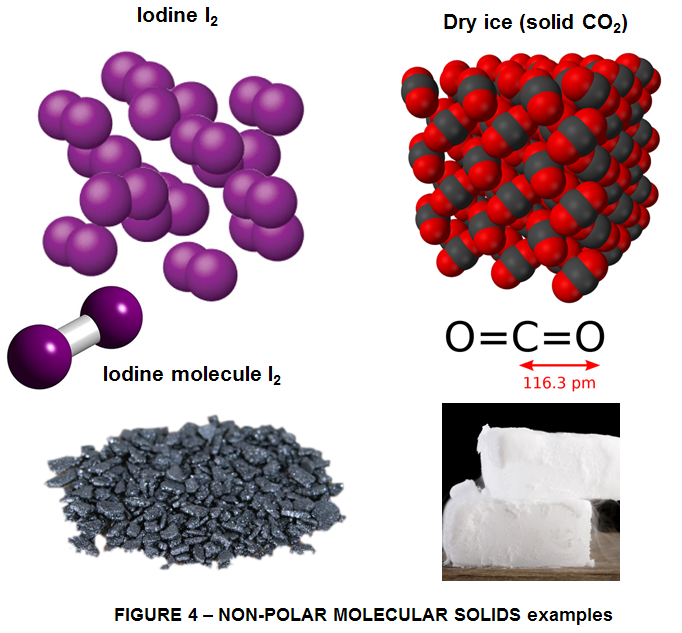
1. Composition:
- Composed of neutral molecules (not ions).
- Held together by IMFs, not covalent bonds between different molecules.
- May include small molecules (e.g., \(\mathrm{CO_2}\)) or large polymers (e.g., polyethylene).
2. Bonding and Forces:
- Intramolecular forces (within each molecule) are strong covalent bonds.
- Intermolecular forces (between molecules) are weak — primarily dispersion or dipole forces.
3. Physical Properties:
Low Melting and Boiling Points: Weak IMFs require little energy to overcome.
Soft and Volatile: Many exist as gases or low-melting solids at room temperature.
Nonconductors: Valence electrons are localized in bonds and lone pairs; no mobile charge carriers.
Types of Molecular Solids by IMF:
| Type of IMF | Examples | Relative Strength | Typical Properties |
|---|---|---|---|
| London Dispersion | \(\mathrm{I_2}\), \(\mathrm{CO_2}\) | Weak | Soft, low melting point, nonconductive |
| Dipole–Dipole | \(\mathrm{SO_2}\), \(\mathrm{CH_3Cl}\) | Moderate | Higher melting/boiling point than dispersion solids |
| Hydrogen Bonding | \(\mathrm{H_2O}\), \(\mathrm{NH_3}\) | Strongest among molecular solids | High melting point for molecular solids, often liquids at room temperature |
Note: Molecular solids are held together by weak intermolecular forces, not covalent or ionic bonds. This leads to low melting and boiling points, softness, and electrical nonconductivity.
Example :
Explain why solid iodine (\(\mathrm{I_2}\)) has a much lower melting point than diamond.
▶️ Answer/Explanation
Step 1: In solid iodine, molecules are held together only by London dispersion forces, which are weak.
Step 2: In diamond, atoms are held together by strong covalent bonds throughout a 3D network.
Final Answer: Diamond’s covalent network requires breaking covalent bonds to melt, while iodine’s weak intermolecular forces require far less energy, resulting in a much lower melting point.
Metallic Solids and Alloys
Metallic solids consist of closely packed metal atoms whose valence electrons are delocalized, forming a “sea of mobile electrons” that moves freely throughout the lattice. This electron mobility accounts for many metallic properties such as electrical conductivity, malleability, ductility, and thermal conductivity.
1. Metallic Bonding Model:
![]()
- Metal atoms contribute valence electrons that become delocalized and free to move throughout the structure.
- Positively charged metal cations (atomic cores) are held together by attraction to this electron sea.
- This explains why metals are both conductive and deformable.
2. Electrical and Thermal Conductivity:
![]()
- Delocalized electrons move freely through the lattice, carrying electric current and thermal energy.
- Metals remain conductive even when alloyed, as long as electron mobility is retained.
3. Malleability and Ductility:
![]()
![]()
- When stress is applied, layers of metal ions can slide past each other without breaking bonds.
- The metallic bond is nondirectional — the electron sea adjusts to the new arrangement, allowing reshaping rather than fracture.
4. Alloys:
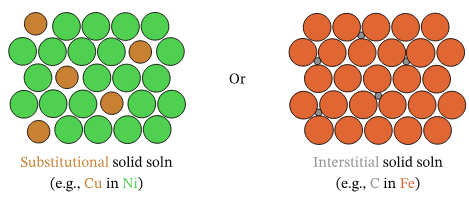
Substitutional Alloys:
Formed when atoms of comparable size substitute for each other in the lattice (e.g., brass: Cu and Zn).
Interstitial Alloys:
Formed when smaller atoms occupy spaces (interstices) between larger metal atoms (e.g., steel: C atoms in Fe lattice). These make the lattice more rigid, reducing malleability and ductility.
- Both types maintain a sea of mobile electrons → remain good electrical conductors.
5. Examples:
| Type | Example | Structure | Properties |
|---|---|---|---|
| Metallic Solid | Copper (Cu) | Close-packed Cu atoms in electron sea | Excellent conductor, malleable, ductile |
| Substitutional Alloy | Brass (Cu–Zn) | Zn replaces Cu atoms | Harder than pure Cu, still conductive |
| Interstitial Alloy | Steel (Fe–C) | C atoms occupy interstices in Fe lattice | Rigid, strong, less ductile |
Note: Metallic solids conduct electricity and heat due to delocalized electrons, and are malleable because the metallic bond is nondirectional. Alloying modifies these properties by changing lattice rigidity and electron distribution but typically preserves conductivity.
Example :
Explain why pure copper is more ductile than steel.
▶️ Answer/Explanation
Step 1: In copper, metallic bonding allows atoms to slide without breaking bonds due to the uniform electron sea.
Step 2: In steel, carbon atoms in interstitial sites disrupt lattice uniformity, reducing the ability of metal ions to move.
Final Answer: The presence of interstitial carbon atoms in steel increases lattice rigidity, making it stronger but less ductile than pure copper.
Noncovalent Interactions in Large Biomolecules and Polymers
In large biomolecules and polymers, noncovalent interactions such as hydrogen bonding, dipole–dipole forces, and dispersion forces occur either between different molecules or within different regions of the same molecule. These weak but numerous interactions determine the molecule’s shape, flexibility, and functionality.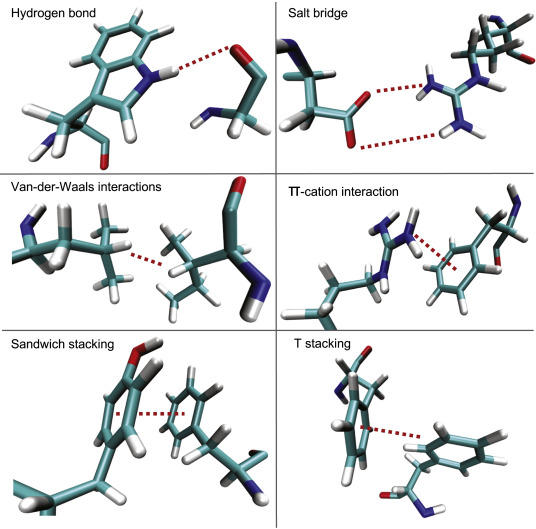
1. Nature of Interactions:
- Noncovalent forces stabilize secondary, tertiary, or quaternary structures in biomolecules (e.g., proteins, DNA).
- These forces also dictate the physical properties of synthetic polymers such as elasticity and melting behavior.
2. Types of Noncovalent Interactions in Large Molecules:
Hydrogen Bonding:
Stabilizes protein α-helices, β-sheets, and DNA base pairs.
Dipole–Dipole and Ion–Dipole Interactions:
Contribute to molecular recognition and binding between charged or polar regions.
London Dispersion Forces:
Provide weak but cumulative attraction between nonpolar regions; important in folding hydrophobic domains.
Hydrophobic Interactions:
Nonpolar segments cluster away from water, helping biomolecules maintain compact structure.
3. Functional Implications:
- Shape determines biological function — enzyme activity, receptor binding, and structural stability depend on precise folding driven by noncovalent forces.
- In polymers, the same interactions influence elasticity, tensile strength, and thermal softening behavior.
Example :
Explain how hydrogen bonding contributes to the double-helix structure of DNA.
▶️ Answer/Explanation
Step 1: Each base pair in DNA is held together by hydrogen bonds between complementary nitrogenous bases (A–T and G–C).
Step 2: Multiple hydrogen bonds collectively stabilize the two DNA strands while allowing them to separate for replication and transcription.
Final Answer: Hydrogen bonding provides structural stability and controlled flexibility to DNA’s double helix, enabling its biological functions.
