AP Chemistry 1.4 Composition of Mixtures Study Notes - New Syllabus 2024-2025
AP Chemistry 1.4 Composition of Mixtures Study Notes- New syllabus
AP Chemistry 1.4 Composition of Mixtures Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
- Explain the quantitative relationship between the elemental composition by mass and the composition of substances in a mixture.
Key Concepts:
- Composition of Mixtures
Composition of Mixtures
Unlike pure substances, which have a fixed composition, mixtures contain two or more substances (elements or compounds) that are physically combined. The composition of a mixture can vary, and the substances retain their own chemical identities.
Key Features of Mixtures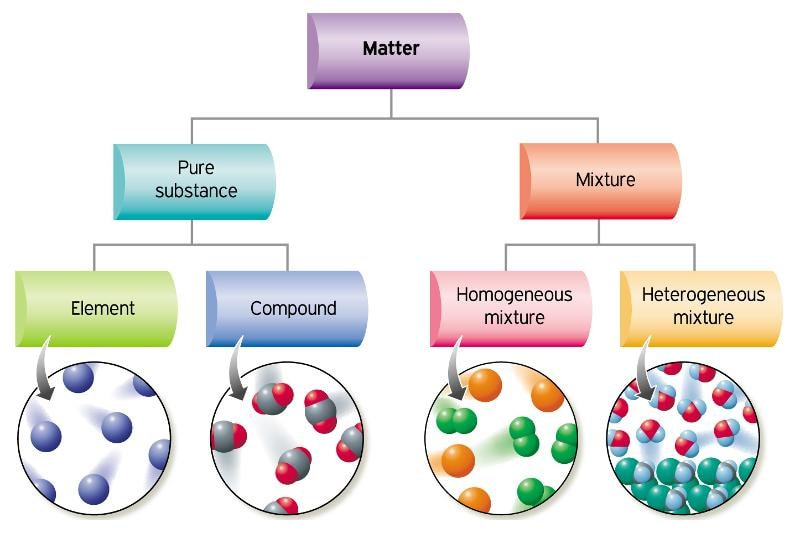
- Components are not chemically bonded.
- Composition can be variable (not fixed like pure substances).
- Separation can be done by physical methods (filtration, distillation, chromatography, etc.).
- Properties of mixtures depend on the proportion of components present.
Quantitative Representation of Mixture Composition
To describe the composition of mixtures, chemists often use:
Mass percent:
\( \% \, \text{Component} = \dfrac{\text{mass of component}}{\text{total mass of mixture}} \times 100 \)
Mole fraction:
\( X_A = \dfrac{n_A}{n_{total}} \)
where \( n_A \) is the number of moles of component A and \( n_{total} \) is the total number of moles of all components.
Parts per million (ppm):
\( \text{ppm} = \dfrac{\text{mass of solute}}{\text{mass of solution}} \times 10^6 \)
Parts per billion (ppb):
\( \text{ppb} = \dfrac{\text{mass of solute}}{\text{mass of solution}} \times 10^9 \)
Mass-to-mass, mass-to-volume, and volume-to-volume ratios are also used depending on the type of mixture.
Example
A mixture contains 20 g of NaCl and 80 g of water. Calculate the mass percent of NaCl in the mixture.
▶️ Answer/Explanation
Total mass = 20 g + 80 g = 100 g
\( \% \, \text{NaCl} = \dfrac{20}{100} \times 100 = 20.0\% \)
Example
A gaseous mixture contains 2 mol of \( \text{O}_2 \), 3 mol of \( \text{N}_2 \), and 1 mol of \( \text{CO}_2 \). Calculate the mole fraction of each gas.
▶️ Answer/Explanation
Total moles = 2 + 3 + 1 = 6
\( X_{O_2} = \dfrac{2}{6} = 0.333 \)
\( X_{N_2} = \dfrac{3}{6} = 0.500 \)
\( X_{CO_2} = \dfrac{1}{6} = 0.167 \)
Example
A water sample contains 0.002 g of lead ions in 1.0 kg of water. Express the concentration in ppm.
▶️ Answer/Explanation
Mass of solution = 1000 g
\( \text{ppm} = \dfrac{0.002}{1000} \times 10^6 = 2.0 \, \text{ppm} \)
