AP Chemistry 1.5 Atomic Structure and Electron Configuration Study Notes - New Syllabus 2024-2025
AP Chemistry 1.5 Atomic Structure and Electron Configuration Study Notes- New syllabus
AP Chemistry 1.5 Atomic Structure and Electron Configuration Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
- Represent the ground-state electron configuration of an atom of an element or its ions using the Aufbau principle.
Key Concepts:
- Atomic Structure and Electron Configuration
Structure of the Atom
An atom consists of a dense, positively charged nucleus containing protons and neutrons, surrounded by electrons moving in regions of space called orbitals.
![]()
| Particle | Charge | Relative Mass | Location |
|---|---|---|---|
| Proton | +1 | ≈ 1 amu | Nucleus |
| Neutron | 0 | ≈ 1 amu | Nucleus |
| Electron | -1 | ≈ 1/1836 amu | Electron cloud (orbitals) |
Atomic Number and Mass Number
![]()
- Atomic number (Z) = number of protons (also equals number of electrons in a neutral atom).
- Mass number (A) = number of protons + neutrons.
- Isotopes = atoms of the same element with the same Z but different A (different numbers of neutrons).
Relative Atomic Mass and Isotopes
The relative atomic mass (\( A_r \)) of an element is the weighted average mass of its naturally occurring isotopes compared to 1/12 the mass of a carbon-12 atom.
\( A_r = \dfrac{\sum (\text{isotopic mass} \times \text{abundance})}{100} \)
Shells, Subshells, and Orbitals
Electrons occupy energy levels (shells), divided into subshells (s, p, d, f) and orbitals:
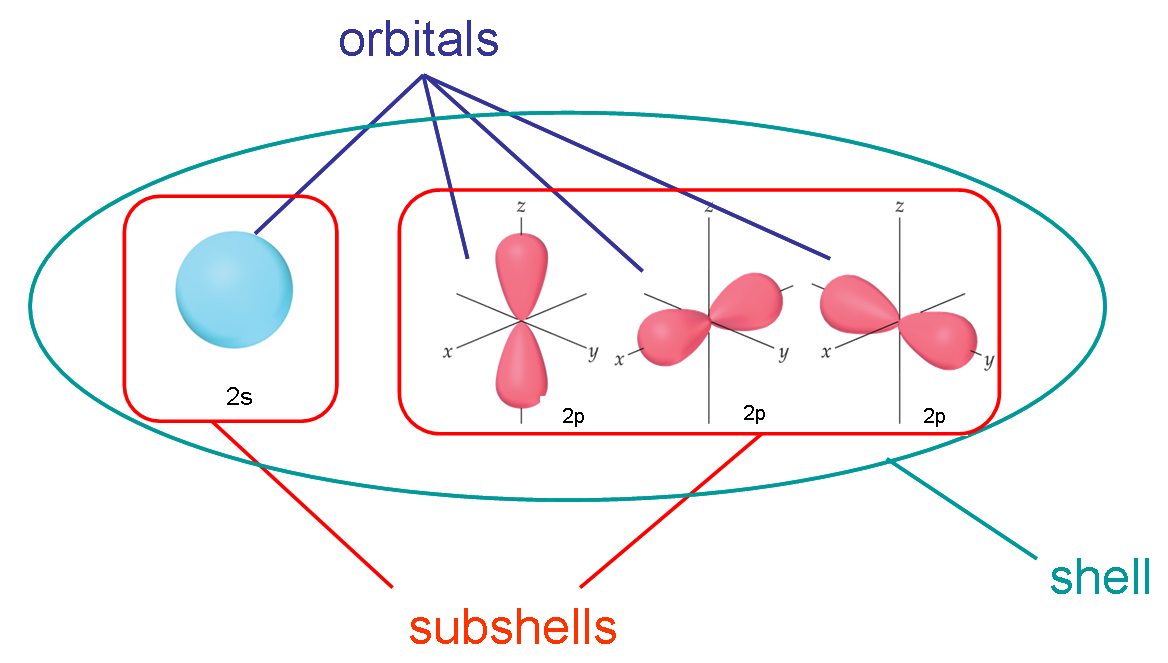
- Shells are labeled by principal quantum number \( n = 1, 2, 3, \dots \).
- Subshells: each shell contains subshells (s, p, d, f).
- Orbitals: regions of space where electrons are most likely found; each orbital holds a maximum of 2 electrons.
Core and Valence Electrons
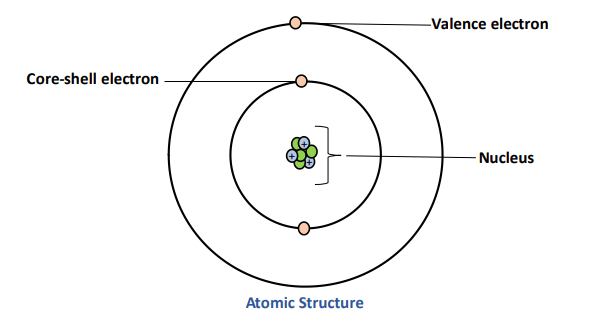
- Core electrons = inner electrons, shield the nucleus.
- Valence electrons = outermost electrons, responsible for bonding and chemical reactivity.
- Periodic properties such as ionization energy, atomic radius, and electronegativity are determined mainly by valence electrons.
Electron Configuration
Electron configuration describes the arrangement of electrons in shells and subshells. It is determined by:
![]()
- Aufbau principle: fill orbitals in order of increasing energy.
- Pauli exclusion principle: no two electrons have the same four quantum numbers; each orbital holds max 2 electrons with opposite spins.
- Hund’s rule: orbitals of the same energy are filled singly before pairing.
Order of Filling:
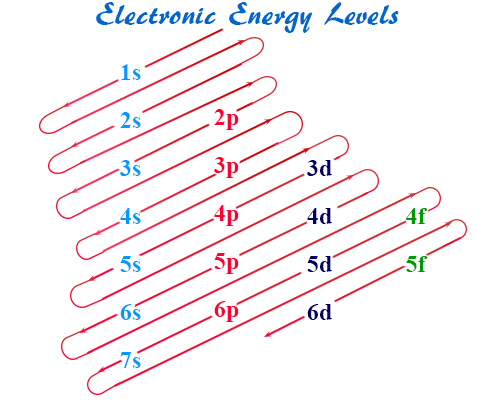
How to Write Electron Configurations
Step 1: Determine the number of electrons (equal to atomic number for a neutral atom).
Step 2: Fill orbitals in the correct order of energy.
Step 3: Apply Pauli Exclusion Principle and Hund’s Rule.
Step 4: Write subshells with superscripts indicating the number of electrons.
Example
Write the electron configuration of oxygen (Z = 8).
▶️ Answer/Explanation
Oxygen has 8 electrons.
Configuration: \( 1s^2 2s^2 2p^4 \)
Core = 2 (in 1s), Valence = 6 (in 2s and 2p).
Example
Write the electron configuration of calcium (Z = 20).
▶️ Answer/Explanation
Calcium has 20 electrons.
Configuration: \( 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 \)
Valence electrons = 2 (in 4s).
Coulomb’s Law and Ionization Energy
The energy required to remove an electron (ionization energy) can be estimated qualitatively using Coulomb’s Law: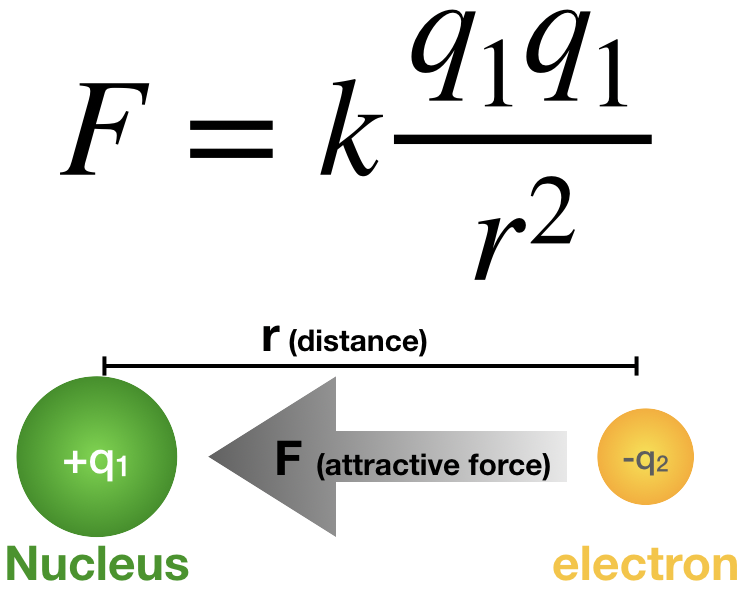
\( F = k \dfrac{q_1 q_2}{r^2} \)
\( q_1 \): effective nuclear charge (\( Z_{eff} \)).
\( q_2 \): charge of the electron.
\( r \): distance between nucleus and electron.
Trends:
- Smaller \( r \) (closer to nucleus) → stronger attraction → higher ionization energy.
- Greater \( Z_{eff} \) → stronger attraction → higher ionization energy.
- More shielding → weaker attraction → lower ionization energy.
Order within a shell: \( s > p > d > f \).
Periodic Table Connection
- Period number = principal quantum number of valence shell.
- Group number = number of valence electrons (for main-group elements).
- Blocks (s, p, d, f) = subshell being filled.
- Thus, the periodic table is a map of electron configurations.
Example
Why is it harder to remove a \( 2s \) electron than a \( 2p \) electron in the same atom?
▶️ Answer/Explanation
The \( 2s \) orbital penetrates closer to the nucleus than the \( 2p \) orbital.
Smaller \( r \) → stronger attraction by nucleus (Coulomb’s Law).
Therefore, \( 2s \) electrons have higher ionization energy than \( 2p \) electrons.
