AP Chemistry 1.6 Photoelectron Spectroscopy- MCQs - Exam Style Questions
Question
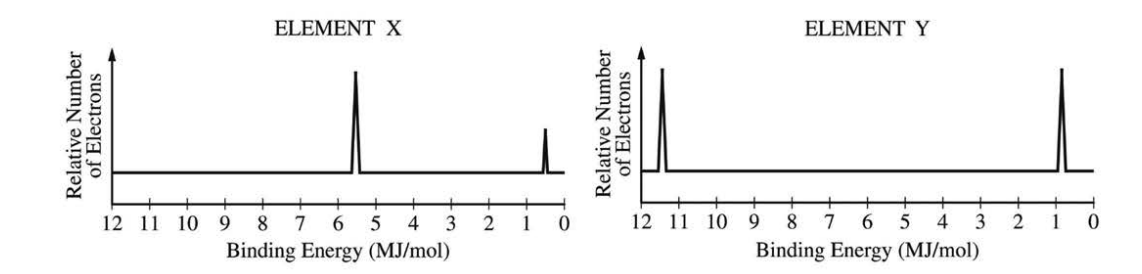 The complete photoelectron spectra of neutral atoms of two unknown elements, X and Y, are shown above.
The complete photoelectron spectra of neutral atoms of two unknown elements, X and Y, are shown above.(B) Element X has a greater ionization energy than element Y does.
(C) Element Y has a greater nuclear charge than element X does.
(D) The isotopes of element Y are approximately equal in abundance, but those of element X are not.
▶️ Answer/Explanation
1. Analyze Photoelectron Spectra (PES):
A PES graph shows the binding energy of electrons in an atom. The peak with the highest binding energy corresponds to the innermost core electrons (the \(1s\) subshell), which are held most tightly by the nucleus.
2. Identify the 1s Peaks:
- For Element X, the innermost peak (highest binding energy) is at approximately \(\sim 5.5\) MJ/mol.
- For Element Y, the innermost peak (highest binding energy) is at approximately \(\sim 11.5\) MJ/mol.
3. Relate Binding Energy to Nuclear Charge:
A higher binding energy for core electrons signifies a stronger electrostatic attraction from the nucleus. This stronger attraction is caused by a greater positive charge in the nucleus (i.e., more protons).
4. Compare the Elements:
Since the \(1s\) binding energy of Element Y (\(\sim 11.5\) MJ/mol) is significantly greater than that of Element X (\(\sim 5.5\) MJ/mol), it can be inferred that Element Y has a greater nuclear charge.
✅ Answer: (C)
Question
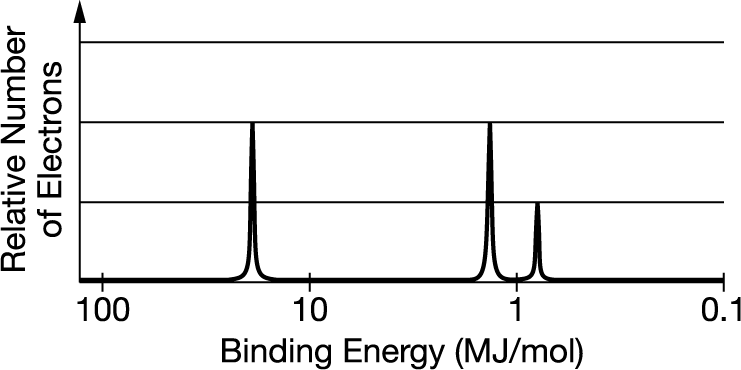
The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom?
A The spectrum shows an odd number electrons.
B The spectrum shows a single electron in the 2p subshell.
C The spectrum shows equal numbers of electrons in the first and second electron shells.
D The spectrum shows three electrons with the same binding energy in the second electron shell.
▶️Answer/Explanation
Ans:B The spectrum is consistent with the electron configuration for boron: \(1s^22^s2^2p^1\). The leftmost peak represents the two electrons in the filled 1s subshell. The two peaks on the right represent the two electrons in the filled 2s subshell and the single electron in the 2p subshell.
