AP Chemistry 1.8 Valence Electrons and Ionic Compounds- MCQs - Exam Style Questions
Question

(B) \( \text{MgO}(s) \) contains no water, but \( \text{MgO}(l) \) contains water that can conduct electricity.
(C) \( \text{MgO}(s) \) consists of separate \( \text{Mg}^{2+} \) ions and \( \text{O}^{2-} \) ions, but \( \text{MgO}(l) \) contains MgO molecules that can conduct electricity.
(D) \( \text{MgO}(s) \) consists of separate \( \text{Mg}^{2+} \) ions and \( \text{O}^{2-} \) ions held in a fixed lattice, but in \( \text{MgO}(l) \) the ions are free to move and conduct electricity.
▶️ Answer/Explanation
1. Understand Electrical Conduction in Ionic Compounds:
Electrical conduction requires mobile charge carriers.
2. Solid vs Liquid State:
– In \( \text{MgO}(s) \), ions are fixed in a crystal lattice and cannot move.
– In \( \text{MgO}(l) \), ions are free to move and can carry electric current.
3. Evaluate Options:
(A) Incorrect – Ionic compounds conduct via ions, not free electrons.
(B) Incorrect – Presence of water is irrelevant for pure \( \text{MgO} \).
(C) Incorrect – \( \text{MgO}(l) \) contains ions, not molecules.
(D) Correct – Describes immobilized vs mobile ions.
✅ Answer: (D)
Questions
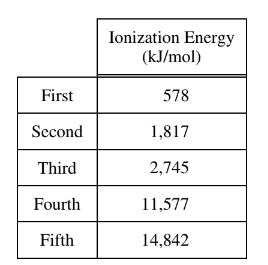
The first five ionization energies of an unknown element are listed in the table above. Which of the following statements correctly identifies the element and cites the evidence supporting the identification?
(A) Na, because of the large difference between the first and the second ionization energies
(B) Al, because of the large difference between the third and fourth ionization energies
(C) Si, because the fifth ionization energy has the greatest value
(D) P, because a neutral atom of P has five valence electrons
▶️Answer/Explanation
Ans: B
(B) Al, because of the large difference between the third and fourth ionization energies:
Aluminum (Al) has 3 valence electrons, so the third ionization energy corresponds to removing the third valence electron, and the fourth ionization energy corresponds to removing the fourth valence electron.
Looking at the data, the jump between the third and fourth ionization energies is indeed quite significant, which is characteristic of the transition from removing valence electrons to removing core electrons.
Therefore, if the answer provided is B, it suggests that the large difference between the third and fourth ionization energies supports the identification of aluminum (Al).
Upon reconsideration, this analysis aligns with the provided answer. So, the correct answer is indeed:
(B) Al, because of the large difference between the third and fourth ionization energies
