AP Chemistry 2.4 Structure of Metals and Alloys- MCQs - Exam Style Questions
Question
| Type of Steel | % Carbon | Characteristics | Uses |
| Low-carbon steel | \(<0.2\%\) | Malleable and ductile | Chains and nails |
| High-carbon steel | \(0.6-1.5\%\) | Hard and brittle | Cutting tools |
(B) The additional carbon atoms within the alloy make the high-carbon steel less dense.
(C) The additional carbon atoms within the alloy increase the thermal conductivity of the high-carbon steel.
(D) The additional carbon atoms within the alloy make it more difficult for the iron atoms to slide past one another.
▶️ Answer/Explanation
1. Understand Metallic Structure:
Metals (like iron) are crystalline solids. Their properties of being malleable and ductile (like low-carbon steel) come from the ability of the layers of metal atoms to slide past one another when a force is applied.
2. Understand the Alloy (Steel):
Steel is an interstitial alloy, where smaller carbon atoms fit into the spaces (interstices) between the larger iron atoms.
3. Compare Low- vs. High-Carbon Steel:
High-carbon steel has more carbon atoms occupying these spaces. These carbon atoms disrupt the uniform layers of iron atoms.
4. Explain Rigidity:
These interstitial carbon atoms act like “pins,” preventing the layers of iron atoms from sliding past each other easily. This resistance to deformation is what makes the material more rigid and hard (and also more brittle, as the table indicates).
✅ Answer: (D)
Question
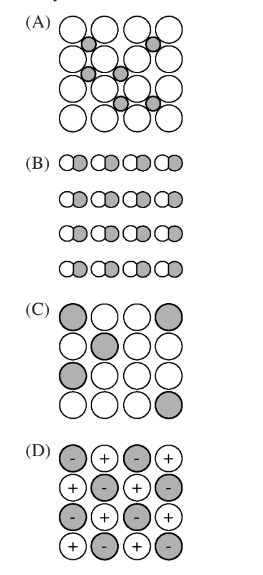
Which of the following diagrams best depicts an alloy of Ni and B ?
▶️Answer/Explanation
Ans:A
The correct diagram that depicts a Ni-B alloy is (A).
Nickel-boron alloys belong to a class of materials known as interstitial alloys. In interstitial alloys, the smaller atoms (in this case, boron) occupy the interstitial sites or voids present within the matrix of larger atoms (nickel).
In diagram (A), the larger circles represent the nickel atoms forming the primary matrix or lattice structure. The smaller dark circles represent the boron atoms that are occupying the interstitial positions or voids between the nickel atoms.
This arrangement, where smaller atoms fit into the interstitial spaces of the larger atom matrix, is characteristic of interstitial alloys like Ni-B. It allows the incorporation of boron atoms into the nickel lattice without severely distorting or disrupting the primary nickel structure.
The other diagrams do not accurately capture this interstitial alloy microstructure. Diagrams (B) and (C) show a random distribution of atoms, not distinguishing the interstitial occupation. Diagram (D) has a different pattern altogether.
Therefore, diagram (A) correctly represents the microstructure of a Ni-B interstitial alloy, with the nickel matrix depicted by larger circles and the interstitially dissolved boron atoms represented by smaller circles within that matrix.
