AP Chemistry 3.1 Intermolecular Forces- MCQs - Exam Style Questions
Question
(B) forces among \(Cl_2\) molecules are stronger than the Cl-Cl bond
(C) Br-Br bond is stronger than the Cl-Cl bond
(D) Cl-Cl bond is stronger than the Br-Br bond
▶️ Answer/Explanation
1. Analyze the States of Matter:
The state of a substance (solid, liquid, or gas) is determined by the strength of its intermolecular forces (IMFs)—the forces between molecules. Stronger IMFs lead to a liquid or solid state, while weaker IMFs lead to a gaseous state at the same temperature.
2. Compare the Substances:
At \(298\) K, \(Br_2\) is a liquid, while \(Cl_2\) is a gas. This means the forces holding \(Br_2\) molecules together are stronger than the forces holding \(Cl_2\) molecules together.
3. Rule out Bond Strength:
Options (B), (C), and (D) refer to the strength of the intramolecular covalent bonds (Br-Br and Cl-Cl). The phase of a substance depends on IMFs, not the strength of the bonds within the molecule.
4. Conclusion:
Because \(Br_2\) is a liquid and \(Cl_2\) is a gas, the intermolecular forces among \(Br_2\) molecules must be stronger than those among \(Cl_2\) molecules.
✅ Answer: (A)
Questions
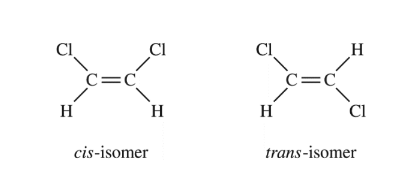
The structural formulas for two isomers of 1,2-dichloroethene are shown above. Which of the two liquids has the higher equilibrium vapor pressure at 20°C, and why?
(A) The cis-isomer, because it has dipole-dipole interactions, whereas the trans-isomer has only London dispersion forces
(B) The cis-isomer, because it has only London dispersion forces, whereas the trans-isomer also has dipole-dipole interactions
(C) The trans-isomer, because it has dipole-dipole interactions, whereas the cis-isomer has only London dispersion forces
(D) The trans-isomer, because it has only London dispersion forces, whereas the cis-isomer also has dipole-dipole interactions
▶️Answer/Explanation
Ans: C
Based on the structural formulas provided for the cis and trans isomers of 1,2-dichloroethene, the correct answer is (C) The trans-isomer, because it has dipole-dipole interactions, whereas the cis-isomer has only London dispersion forces.
In the trans isomer, the two chlorine atoms are on opposite sides of the C=C double bond. This results in a net dipole moment for the molecule due to the electronegativity difference between carbon and chlorine atoms. The trans isomer molecules can experience dipole-dipole interactions in addition to London dispersion forces.
In the cis isomer, however, the two chlorine atoms are on the same side of the C=C double bond. This results in a net zero dipole moment as the dipole moments from the two C-Cl bonds cancel out. The cis isomer molecules only experience London dispersion forces between the instantaneous dipoles.
Dipole-dipole interactions are generally weaker than covalent or ionic bonds but stronger than London dispersion forces. The presence of these dipole-dipole interactions in the trans isomer requires more energy to overcome compared to just London forces in the cis isomer.
Therefore, the trans isomer with dipole-dipole interactions will have a lower vapor pressure at equilibrium compared to the cis isomer which exhibits only London dispersion forces. Substances with weaker intermolecular forces have higher vapor pressures.
Questions
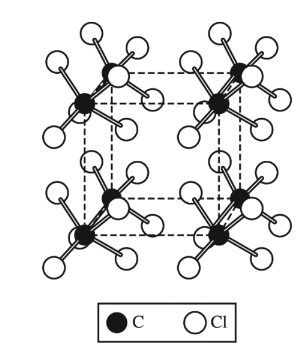
Solid carbon tetrachloride, \(CCl_4\)(s), is represented by the diagram above. The attractions between the\( CCl_4\) molecules that hold the molecules
together in the solid state are best identified as
(A) polar covalent bonds
(B) nonpolar covalent bonds
(C) intermolecular attractions resulting from temporary dipoles
(D) intermolecular attractions resulting from permanent dipoles
▶️Answer/Explanation
Ans: C
Carbon tetrachloride (\(CCl_4\)) is a nonpolar molecule due to the symmetrical arrangement of its four chlorine atoms around the central carbon atom, which results in a net zero dipole moment.
In the solid state, the interactions between \(CCl_4\) molecules are primarily due to intermolecular forces rather than intramolecular bonds because \(CCl_4\) molecules do not form covalent bonds with each
other.
The correct answer is:
(C) Intermolecular attractions resulting from temporary dipoles
These temporary dipoles arise from the movement of electrons within molecules, leading to temporary imbalances in electron distribution, which induce temporary dipoles in neighboring molecules. This type
of intermolecular attraction is known as London dispersion forces or van der Waals forces.
