AP Chemistry 3.11 Spectroscopy and the Electromagnetic Spectrum- MCQs - Exam Style Questions
Question
\( \text{Ne}, \text{HF}, \text{C}_2\text{H}_6, \text{CH}_4 \)
Which of the substances listed above has the highest boiling point, and why?
(B) HF, because its molecules form hydrogen bonds
(C) \( \text{C}_2\text{H}_6 \), because each molecule can form multiple hydrogen bonds
(D) \( \text{CH}_4 \), because its molecules have the greatest London dispersion forces
▶️ Answer/Explanation
1. Intermolecular Forces and Boiling Points:
Hydrogen bonding > dipole-dipole > London dispersion
2. Analyze Each Compound:
– Ne: Only London dispersion (very weak)
– HF: Hydrogen bonding (strong)
– \( \text{C}_2\text{H}_6 \): Only London dispersion
– \( \text{CH}_4 \): Only London dispersion
3. Evaluate Options:
(B) Correct – HF has hydrogen bonding, giving it the highest boiling point.
✅ Answer: (B)
Questions
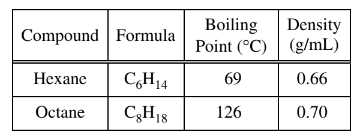
A student obtains a mixture of the liquids hexane and octane, which are miscible in all proportions. Which of the following techniques would be best for separating the two components of the mixture, and why?
(A) Filtration, because the different densities of the liquids would allow one to pass through the filter paper while the other would not.
(B) Paper chromatography, because the liquids would move along the stationary phase at different rates owing to the difference in polarity of their molecules.
(C) Column chromatography, because the higher molar mass of octane would cause it to move down the column faster than hexane.
(D) Distillation, because the liquids would boil at different temperatures owing to the difference in strength of their intermolecular forces.
▶️Answer/Explanation
Ans: D
To separate a mixture of hexane and octane, both of which are volatile hydrocarbons with similar chemical properties but different boiling points, the most effective technique would be based on the difference
in their boiling points. Let’s analyze the options:
(A) Filtration: Filtration is typically used to separate a solid from a liquid or a precipitate from a solution. However, hexane and octane are both liquids, and they are miscible (mixable) in all proportions.
Their density difference is minimal and not sufficient for effective separation.
(B) Paper chromatography: Paper chromatography separates components based on differences in polarity. While hexane and octane have different boiling points, they have similar polarities since they are
both nonpolar hydrocarbons. Thus, paper chromatography would not effectively separate them.
(C) Column chromatography: Column chromatography separates compounds based on differences in their affinity for a stationary phase (usually silica gel) and their interaction with a mobile phase
(solvent). While octane has a higher molar mass than hexane, their similar chemical properties and nonpolar nature make them unlikely to separate effectively using column chromatography.
(D) Distillation: Distillation is the process of separating components of a mixture based on differences in their boiling points. Hexane has a lower boiling point (69C) compared to octane (126C) due to
differences in the strength of their intermolecular forces. Therefore, distillation would be the most suitable technique to separate hexane and octane effectively.
So, the correct answer is:
(D) Distillation, because the liquids would boil at different temperatures owing to the difference in strength of their intermolecular forces.
Questions
\(Cu_{(s)}+4HNO_{3}(aq)\rightarrow Cu(No_{3})_{2}(aq)+2No_{2(g)}+2H_{2}o_{(l)}\)
Each student in a class placed a \(2.00 \mathrm{~g}\) sample of a mixture of \(\mathrm{Cu}\) and \(\mathrm{Al}\) in a beaker and placed the beaker in a fume hood. The students slowly poured \(15.0 \mathrm{~mL}\) of \(15.8 \mathrm{M} \mathrm{HNO}_3(a q)\) into their beakers. The reaction between the copper in the mixture and the \(\mathrm{HNO}_3(a q)\) is represented by the equation above. The students observed that a brown gas was released from the beakers and that the solutions turned blue, indicating the formation of \(\mathrm{Cu}^{2+}(a q)\). The solutions were then diluted with distilled water to known volumes.

To determine the number of moles of Cu in the sample of the mixture, the students measured the absorbance of known concentrations of Cu(NO3)2(aq) using a spectrophotometer. A cuvette filled with some of the solution produced from the sample of the mixture was also tested. The data recorded by one student are shown in the table above. On the basis of the data provided, which of the following is a possible error that the student made?
(A) The Cu(NO3)2(aq) from the sample of the mixture was not diluted properly.
(B) The spectrophotometer was calibrated with tap water instead of distilled water.
(C) The student labeled the cuvettes incorrectly, reversing the labels on two of the solutions of known concentration.
(D) The spectrophotometer was originally set to an inappropriate wavelength, causing the absorbance to vary unpredictably.
▶️Answer/Explanation
Ans: C
- option \(C\) is correct because the rise in absorbance is linearly increasing but the labels are not correct.

The correct order is.

- With rise in concenterat absorbance increases.
