AP Chemistry 4.4 Physical and Chemical Changes - MCQs - Exam Style Questions
Question


(B)

(C)
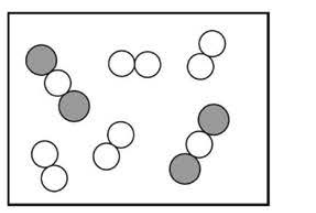
(D)

▶️ Answer/Explanation
1. Reaction Stoichiometry:
\( 2\text{CO} + \text{O}_2 \rightarrow 2\text{CO}_2 \)
The reaction consumes \( 2 \) CO molecules for every \( 1 \) O₂ molecule.
2. Analyze Initial Mixture:
From the diagram, there are more O₂ molecules than needed to react with all CO.
3. Determine Limiting Reactant:
CO is the limiting reactant; O₂ is in excess.
After reaction: Only CO₂ and leftover O₂ remain.
✅ Answer: (B)
Question
A student combines a solution of \(NaCl\)(aq) with a solution of \(AgNO_3\)(aq) , and a precipitate forms. Which of the following is evidence that ionic bonds formed during the precipitation?
A The resulting solution is colorless.
B The resulting solution conducts electricity.
C The precipitate has a high melting point.
D The temperature of the solution did not change significantly during the precipitation.
▶️Answer/Explanation
Ans:C
A characteristic of compounds containing ionic bonding is a high melting point.
