Ap Chemistry 4.8 Introduction to Acid - Base Reactions- MCQs - Exam Style Questions
Question

▶️ Answer/Explanation
1. Analyze Reactants (Lewis Structures):
- \(NH_{3}\) (Ammonia): The central nitrogen atom has \(5\) valence electrons. It forms \(3\) single bonds with hydrogen and has one lone pair of electrons. This makes it a Lewis base (an electron-pair donor).
- \(BF_{3}\) (Boron trifluoride): The central boron atom has \(3\) valence electrons. It forms \(3\) single bonds with fluorine. It has an incomplete octet (\(6\) electrons) and an empty \(p\)-orbital. This makes it a Lewis acid (an electron-pair acceptor).
2. Predict the Reaction (Lewis Acid-Base):
A Lewis acid-base reaction occurs. The lone pair on the nitrogen (\(N\)) of \(NH_{3}\) is donated to the empty orbital of the boron (\(B\)) of \(BF_{3}\). This forms a new coordinate covalent bond between the nitrogen and boron atoms.
3. Evaluate the Options:
- (A) and (B) incorrectly show a hydrogen bond between the molecules.
- (C) incorrectly shows an N-F bond.
- (D) correctly shows the \(NH_{3}\) molecule and the \(BF_{3}\) molecule joined by a new, single N-B bond.
✅ Answer: (D)
Questions
Samples of NaF(s) and\( NH_{4}\)Cl(s) are dissolved in separate beakers that each contain 100 mL of water. One of the salts produces a slightly acidic solution. Which of the following equations best represents the formation of the slightly acidic solution?
(A) $Na +(aq) + 2 H_{2}O(l) \rightleftharpoons NaOH(aq) + H_{3}O^{+}$(aq)
(B) $F ^{−}(aq) + H_{2}O(l) \rightleftharpoons HF(aq) + OH^{−}$(aq)
(C) $NH_{ 4}+(aq) + H_{2}O(l) \rightleftharpoons NH_3(aq) + H_{3}O^{+}$(aq)
(D)$Cl ^{−}(aq) + H_{2}O(l) \rightleftharpoons HCl(aq) + OH^{−}$(aq)
▶️Answer/Explanation
Ans: C
The correct answer is C. When \(NH_{4}^{+}\) ions are dissolved in water, they can act as a Bronsted-Lowry acid and donate a proton \(H^+\) to water molecules, forming \(NH_{3}(aq)\) and \(H_{3}O^{+}
(aq)\). This increases the concentration of \(H_{3}O^{+}\) ions in the solution, making it slightly acidic. Here is the reaction:
\(NH_{ 4}+(aq) + H_{2}O(I) \rightleftharpoons NH3(aq) + H_{3}0^{+}\)(aq)
This process is known as the hydrolysis of \(NH_{4}^{+}\) ions.
Question
\(C_6H_5COOH(aq)+NaOH(aq)→C_6H_5COONa(aq)+H2O(l)\)
Which of the following identifies a conjugate acid-base pair in the reaction represented above?
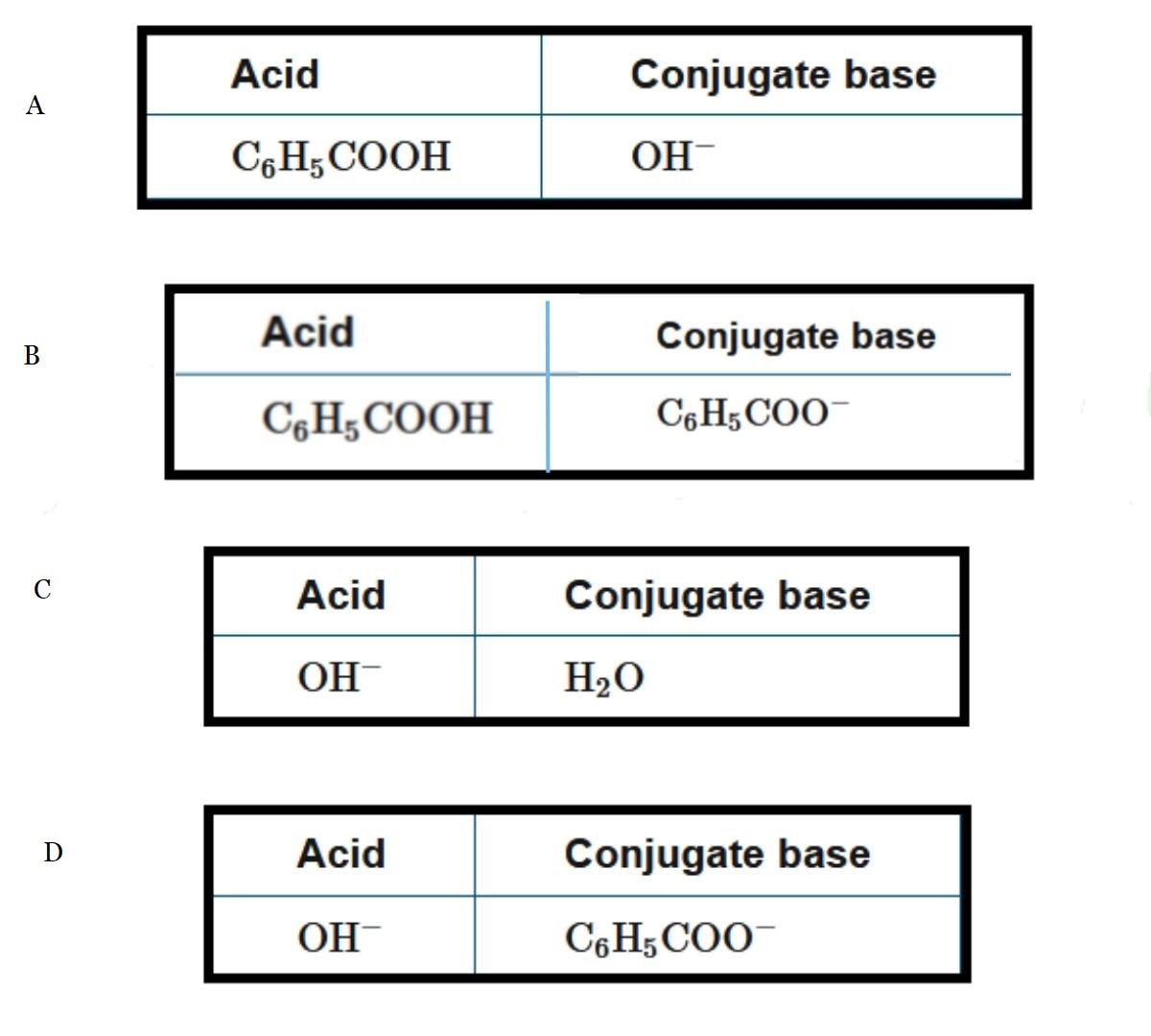
▶️Answer/Explanation
Ans: B
The conjugate base is the species that results from the loss of the proton \(H^+\) from the acid. In this reaction, \(C_6H_5COOH\) is a weak acid that donates an \(H^+\) to \(OH^−\) to form \(H_2O\) and the conjugate base, \(C_6H_5COO^−\).
