AP Chemistry 5.1 Reaction Rates Study Notes - New Syllabus Effective fall 2024
AP Chemistry 5.1 Reaction Rates Study Notes- New syllabus
AP Chemistry 5.1 Reaction Rates Study Notes – AP Chemistry – per latest AP Chemistry Syllabus.
LEARNING OBJECTIVE
Explain the relationship between the rate of a chemical reaction and experimental parameters.
Key Concepts:
- Reaction Kinetics
- Relationship Between Reaction Rate and Stoichiometry
- Factors Affecting the Rate of a Chemical Reaction
Reaction Kinetics
Chemical kinetics is the study of the rate at which reactants are converted into products during a chemical reaction. The rate of a reaction expresses how fast the concentration of a reactant or product changes per unit time.
Mathematical Expression: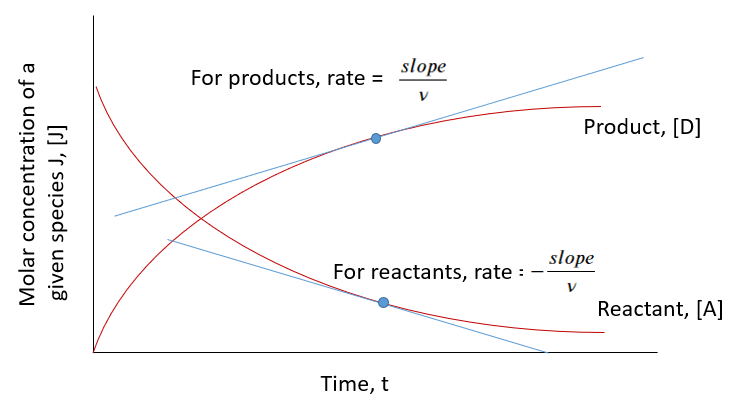
\( \mathrm{Rate = \dfrac{-\Delta [Reactant]}{\Delta t} = \dfrac{+\Delta [Product]}{\Delta t}} \)
- \( \mathrm{[Reactant]} \): concentration of a reactant (mol/L)
- \( \mathrm{[Product]} \): concentration of a product (mol/L)
- \( \mathrm{\Delta t} \): time interval (s)
- Negative sign indicates that reactant concentration decreases over time.
Units of Rate: \( \mathrm{mol\,L^{-1}\,s^{-1}} \) (or \( \mathrm{M/s} \))
Reaction kinetics quantifies how quickly reactants are consumed or products are formed, helping to understand both mechanism and control of chemical reactions.
Example
The concentration of \( \mathrm{H_2} \) gas in a reaction decreases from \( \mathrm{0.100\,mol/L} \) to \( \mathrm{0.040\,mol/L} \) in \( \mathrm{20\,s.} \) Calculate the average rate of reaction with respect to \( \mathrm{H_2.} \)
▶️ Answer / Explanation
Step 1: Use the rate formula:
\( \mathrm{Rate = -\dfrac{\Delta [H_2]}{\Delta t}} \)
Step 2: Substitute the values:
\( \mathrm{Rate = -\dfrac{(0.040 – 0.100)}{20} = \dfrac{0.060}{20} = 3.0\times10^{-3}\,mol\,L^{-1}\,s^{-1}} \)
Step 3: Interpret the result:
The average rate of disappearance of \( \mathrm{H_2} \) is \( \mathrm{3.0\times10^{-3}\,M/s.} \)
Result: The reaction consumes hydrogen gas at a rate of \( \mathrm{3.0\times10^{-3}\,mol\,L^{-1}\,s^{-1}.} \)
Relationship Between Reaction Rate and Stoichiometry
The rates of change of reactant and product concentrations in a chemical reaction are related by the stoichiometric coefficients in the balanced chemical equation. Each species changes at a rate proportional to its coefficient in the reaction.
General Representation: 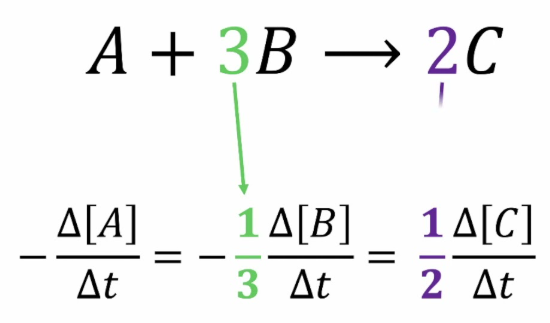
For a reaction: \( \mathrm{aA + bB \rightarrow cC + dD} \)
\( \mathrm{Rate = -\dfrac{1}{a}\dfrac{\Delta [A]}{\Delta t} = -\dfrac{1}{b}\dfrac{\Delta [B]}{\Delta t} = \dfrac{1}{c}\dfrac{\Delta [C]}{\Delta t} = \dfrac{1}{d}\dfrac{\Delta [D]}{\Delta t}} \)
- Negative signs (−) for reactants indicate decreasing concentration.
- Positive signs (+) for products indicate increasing concentration.
- The coefficients \( \mathrm{a, b, c, d} \) normalize the rate for each species so the overall reaction rate is consistent.
The rate of reaction is the same regardless of whether it’s measured using a reactant or a product — stoichiometric ratios ensure consistency among all species.
Example
For the reaction
\( \mathrm{2NO(g) + O_2(g) \rightarrow 2NO_2(g)} \)
if \( \mathrm{[O_2]} \) is decreasing at a rate of \( \mathrm{0.020\,mol\,L^{-1}\,s^{-1}} \), determine:
- (a) The rate of disappearance of \( \mathrm{NO} \)
- (b) The rate of appearance of \( \mathrm{NO_2} \)
▶️ Answer / Explanation
Step 1: Write the stoichiometric rate relationship.
\( \mathrm{-\dfrac{1}{2}\dfrac{\Delta [NO]}{\Delta t} = -\dfrac{1}{1}\dfrac{\Delta [O_2]}{\Delta t} = \dfrac{1}{2}\dfrac{\Delta [NO_2]}{\Delta t}} \)
Step 2: Use the given rate of \( \mathrm{O_2} \):
\( \mathrm{\dfrac{\Delta [O_2]}{\Delta t} = -0.020\,mol\,L^{-1}\,s^{-1}} \)
Step 3: Find rate for \( \mathrm{NO} \):
From the stoichiometry, \( \mathrm{\dfrac{\Delta [NO]}{\Delta t} = 2 \times \dfrac{\Delta [O_2]}{\Delta t}} \)
\( \mathrm{\dfrac{\Delta [NO]}{\Delta t} = 2 \times (-0.020) = -0.040\,mol\,L^{-1}\,s^{-1}} \)
Rate of disappearance of \( \mathrm{NO} = 0.040\,mol\,L^{-1}\,s^{-1}. \)
Step 4: Find rate for \( \mathrm{NO_2} \):
From the stoichiometry, \( \mathrm{\dfrac{\Delta [NO_2]}{\Delta t} = 2 \times 0.020 = 0.040\,mol\,L^{-1}\,s^{-1}} \)
Result:
- (a) \( \mathrm{NO} \) disappears at \( \mathrm{0.040\,mol\,L^{-1}\,s^{-1}} \)
- (b) \( \mathrm{NO_2} \) forms at \( \mathrm{0.040\,mol\,L^{-1}\,s^{-1}} \)
This shows that stoichiometric relationships maintain consistent overall reaction rate.
Factors Affecting the Rate of a Chemical Reaction
The rate of a chemical reaction depends on several factors that influence how often and how effectively reactant particles collide. These factors include reactant concentration, temperature, surface area, catalysts, and other environmental conditions such as pressure (for gases) and solvent polarity (for solutions).
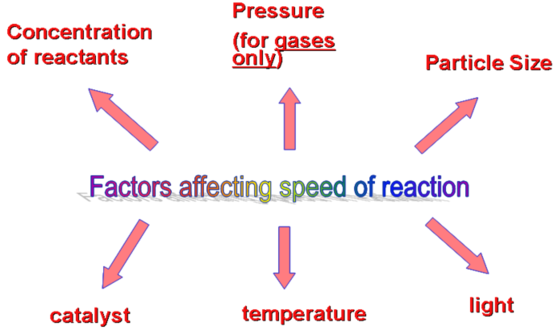
Each factor affects either the frequency of collisions or the energy of particles — both of which determine how quickly products form according to the collision theory.
Major Factors Affecting Reaction Rate:
| Factor | Effect on Rate | Explanation |
|---|---|---|
| 1. Reactant Concentration | ↑ Concentration → ↑ Rate | Higher concentration means more frequent collisions between reactant molecules, increasing the likelihood of effective collisions. |
| 2. Temperature | ↑ Temperature → ↑ Rate | Higher temperature increases particle kinetic energy, so a greater fraction of collisions have energy ≥ activation energy (\( \mathrm{E_a} \)). |
| 3. Surface Area | ↑ Surface Area → ↑ Rate | More exposed surface allows more collisions to occur simultaneously (important for solids reacting with gases or liquids). |
| 4. Catalysts | Presence of catalyst → ↑ Rate | A catalyst lowers the activation energy by providing an alternate reaction pathway without being consumed in the reaction. |
| 5. Nature of Reactants | Varies | Some substances react faster because their bonds break more easily or because they exist in ionic rather than covalent form. |
Relevant Relation (Arrhenius Equation):
\( \mathrm{k = A e^{-\dfrac{E_a}{RT}}} \)
- \( \mathrm{k} \): rate constant
- \( \mathrm{A} \): frequency factor (collision frequency & orientation)
- \( \mathrm{E_a} \): activation energy (J/mol)
- \( \mathrm{R} \): gas constant (\( \mathrm{8.314\,J\,mol^{-1}K^{-1}} \))
- \( \mathrm{T} \): absolute temperature (K)
Increasing temperature exponentially increases the rate constant \( \mathrm{k} \) by increasing the number of particles with sufficient energy to overcome \( \mathrm{E_a} \).
Example
Hydrogen peroxide (\( \mathrm{H_2O_2} \)) decomposes into water and oxygen gas according to:
\( \mathrm{2H_2O_2(aq) \rightarrow 2H_2O(l) + O_2(g)} \)
It is observed that the decomposition is very slow at room temperature but becomes rapid when the solution is heated or when manganese dioxide (\( \mathrm{MnO_2} \)) is added. Explain why.
▶️ Answer / Explanation
Step 1: Identify relevant factors — temperature and catalyst.
Step 2: Effect of temperature:
Heating increases the average kinetic energy of \( \mathrm{H_2O_2} \) molecules, so more collisions have energy greater than the activation energy. This increases the rate of decomposition.
Step 3: Effect of catalyst (\( \mathrm{MnO_2} \)):
\( \mathrm{MnO_2} \) provides an alternate reaction pathway with lower activation energy (\( \mathrm{E_a} \)), allowing the reaction to proceed faster even at lower temperatures.
Step 4: Final explanation:
At higher temperature and with a catalyst, the reaction rate increases because more molecules possess sufficient energy for successful collisions.
Result: The decomposition of \( \mathrm{H_2O_2} \) is much faster when heated or when \( \mathrm{MnO_2} \) is added due to increased collision effectiveness and reduced activation energy.
