AP Chemistry 5.2 Introduction to Rate Law - MCQs - Exam Style Questions
Question
colorless brown
\(K_{p}=3.0\) at \(70^{\circ}C\)
A mixture of \(NO_{2}(g)\) and \(N_{2}O_{4}(g)\) is placed in a glass tube and allowed to reach equilibrium at \(70^{\circ}C\), as represented above.
(B) \(2.0\) atm
(C) \(2.3\) atm
(D) \(4.0\) atm
▶️ Answer/Explanation
1. Write the \(K_p\) Expression:
The equilibrium constant expression for the given reaction is:
\(K_p = \frac{(P_{NO_{2}})^2}{P_{N_{2}O_{4}}}\)
2. Substitute Known Values:
We are given \(K_p = 3.0\) and \(P_{N_{2}O_{4}} = 1.33\) atm.
\(3.0 = \frac{(P_{NO_{2}})^2}{1.33}\)
3. Solve for \((P_{NO_{2}})^2\):
Multiply both sides by \(1.33\):
\((P_{NO_{2}})^2 = 3.0 \times 1.33\)
\((P_{NO_{2}})^2 = 3.99\)
4. Solve for \(P_{NO_{2}}\):
Take the square root of both sides:
\(P_{NO_{2}} = \sqrt{3.99} \approx 2.0\) atm
✅ Answer: (B)
Questions
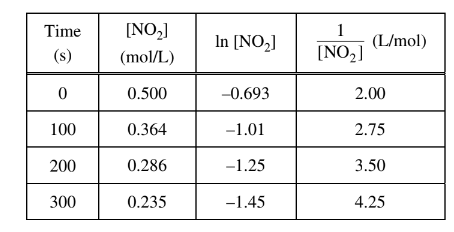
The data from a study of the decomposition of \(NO_2\)(g) to form NO(g) and \(O_2\)(g) are given in the table above. Which of the following rate laws is consistent with the data?
(A) Rate = \(k[NO_{2}]\)
(B) Rate=\(k[NO_{2}]^{2}\)
(C) Rate=\(K\frac{1}{[NO_{2}]}\)
(D) Rate= \(K\frac{1}{[NO_{2}]^{2}}\)
▶️Answer/Explanation
Ans: A
To determine the rate law from the given data, we can utilize the integrated rate laws for first-order and second-order reactions.
For a first-order reaction, the integrated rate law is:
\[ \ln{[\text{NO}_2]} = -kt + \ln{[\text{NO}_2]_0} \]
For a second-order reaction, the integrated rate law is:
\[ \frac{1}{[\text{NO}_2]} = kt + \frac{1}{[\text{NO}_2]_0} \]
Comparing the given data with the integrated rate laws, we can identify the order of the reaction.
Looking at the data, when the concentration of \(NO_2\) decreases, the natural logarithm of \(NO_2\) increases linearly with time. This behavior is consistent with a first-order reaction.
Therefore, the rate law for a first-order reaction is:
\[ \text{Rate} = k[\text{NO}_2]\]
So, the correct answer is: (A) Rate =\(k[NO_{2}]\)
Question
\(2NOBr(g)\rightarrow 2NO(g)+Br_{2}(g)\)
The equation above represents an elementary step in a chemical reaction. Which of the following is the correct expression for the rate law of the elementary step?
(A) Rate=k[NOBr]^{\frac{1}{2}}
(B) Rate=k[NOBr]
(C) Rate= \(k[NOBr]^{2}\)
(D) Rate= \(k[NO]^{2}[Br_{2}]\)
▶️Answer/Explanation
Ans:C
The given equation is:
\[ 2NOBr(g) \rightarrow 2NO(g) + Br_2(g) \]
From the balanced equation, we can see that the reactant is \(NOBr\). The stoichiometric coefficient of \(NOBr\) in the balanced equation is 1. Therefore, the rate law expression will depend on the concentration of \(NOBr\).
However, this is a bimolecular reaction, meaning two molecules of \(NOBr\) are involved in the rate-determining step. Therefore, the rate law expression will include \(NOBr\) raised to the power of 2.
So, the correct expression for the rate law of the elementary step is:
(C) Rate = \(k[NOBr]^2\)
