AP Chemistry 5.5 Collision Model - MCQs - Exam Style Questions
Question
(B) The activation energy of the reaction will decrease.
(C) The frequency of collisions between \(H^{+}(aq)\) ions and \(ClO^{-}(aq)\) ions will increase.
(D) The value of the rate constant will increase.
▶️ Answer/Explanation
1. Identify the Role of \(H^{+}\):
In the given reaction, \(H^{+}(aq)\) is a reactant.
2. Apply Collision Theory:
According to collision theory, the rate of a chemical reaction is proportional to the frequency of effective collisions between reactant particles.
3. Analyze the Effect of Concentration:
Increasing the concentration of a reactant (increasing \([H^{+}]\)) means there are more \(H^{+}\) ions in the same volume. This increases the probability of these ions colliding with other reactant particles, namely \(Cl^{-}(aq)\) and \(ClO^{-}(aq)\).
4. Evaluate the Options:
- (A) and (B) are incorrect. Activation energy is changed by a catalyst, not by reactant concentration.
- (D) is incorrect. The rate constant (\(k\)) is dependent on temperature, not concentration.
- (C) is correct. Increasing the concentration of \(H^{+}\) directly increases the frequency of collisions between \(H^{+}\) and all other reactants, including \(ClO^{-}\).
✅ Answer: (C)
Question
NO(g) + \(NO_3\)(g) → 2 \(NO_2\)(g)
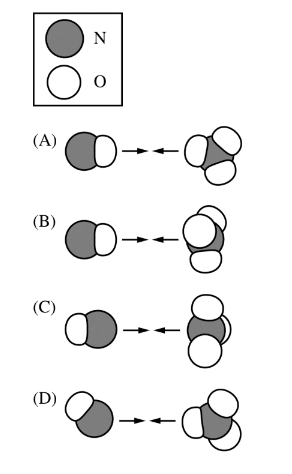
The reaction between NO(g) and \(NO_3\)(g) is represented by the equation above. Which of the following orientations of collision between NO(g) and \(NO_3\)(g) is most likely to be effective?
▶️Answer/Explanation
Ans:D
For the reaction between NO(g) and \(NO_3(g)\) to produce \(2NO_2(g)\), the most effective collision orientation would be one that allows the reactants to form the products most efficiently. This typically involves the approach of reactive parts of the molecules towards each other.
Based on the image description, here are the considerations for each orientation:
Orientation A: The nitrogen atom of NO colliding with an oxygen atom of \(NO_3\) might not be the most effective because it doesn’t directly lead to the formation of \(NO_2\).
Orientation B: The oxygen atom of NO colliding with an oxygen atom of \(NO_3\) is also less likely to be effective for the same reason as A.
Orientation C: The nitrogen atom of NO colliding with the nitrogen atom of \(NO_3\) could be effective if it leads to the formation of two \(NO_2\) molecules.
Orientation D: The oxygen atom of NO colliding with the nitrogen atom of \(NO_3\) seems the most likely to be effective, as it could facilitate the transfer of an oxygen atom to form two \(NO_2\) molecules.
Therefore, Orientation D is most likely to be the effective collision orientation for this reaction, as it aligns with the expected product formation.
Question
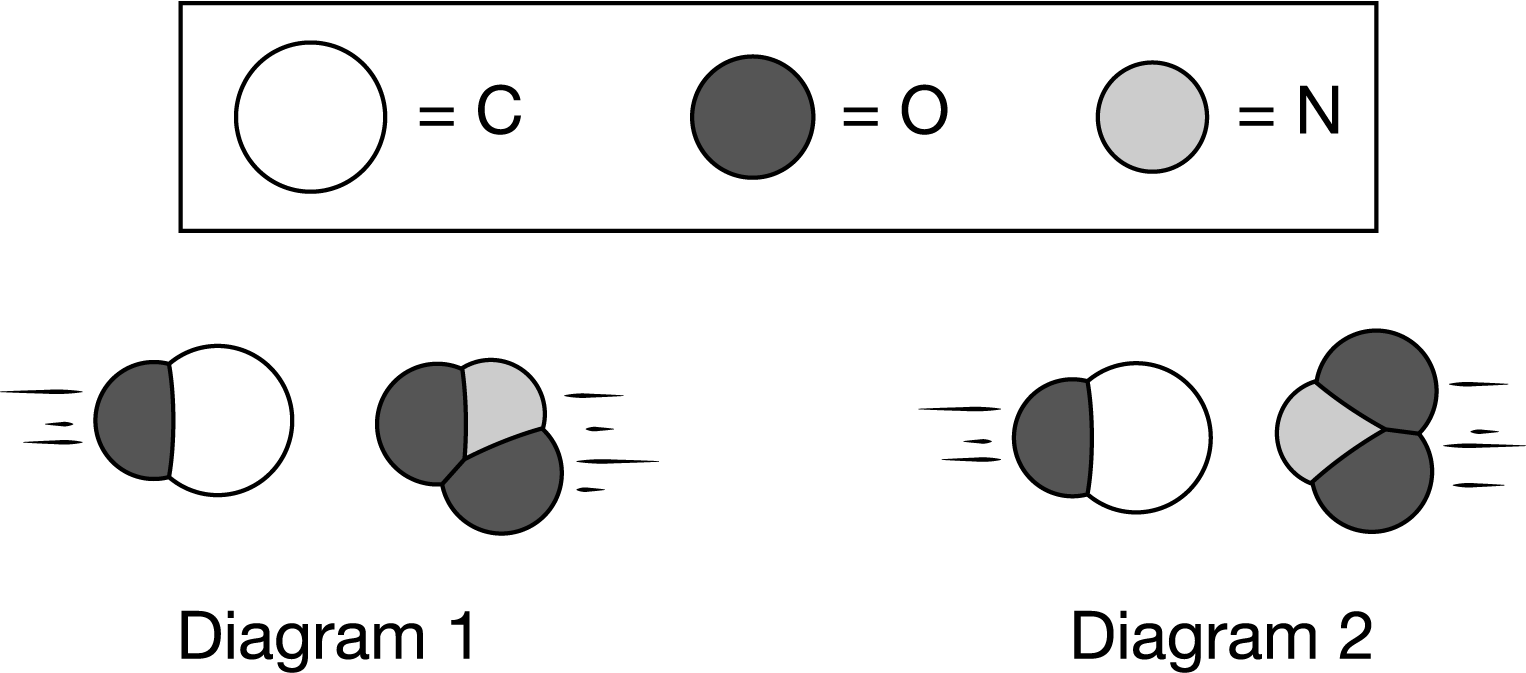
The two diagrams above represent collisions that take place at the same temperature between a CO molecule and an \(NO_2\) molecule. The products are \(CO_2\) and NO. Which diagram most likely represents an effective collision, and why?
A Diagram 1 represents an effective collision because the molecules have about the same size and the same energy, which leads to a larger rate constant (k).
B Diagram 1 represents an effective collision because the two molecules have the proper orientation to form a new C−O bond as long as they possess enough energy to overcome the activation energy barrier.
C Diagram 2 represents an effective collision because the molecules are aligned head-to-head and that maximizes the overlap between their atomic orbitals, forming more products.
D Diagram 2 represents an effective collision because the oxygen atoms are the most electronegative and will minimize their repulsions when oriented away from each other.
▶️Answer/Explanation
Ans: B
In order for this reaction to form \(CO_2\) and NO, the reactants CO and \(NO_2\) must be oriented in a way that allows for a bond between N and O to be broken while a new bond between C and O forms. Diagram 1 represents the best orientation for an effective collision as long as the molecules have the minimum energy required to overcome the activation energy barrier.
