AP Chemistry 5.7 Introduction to Reaction Mechanisms - MCQs - Exam Style Questions
Question
The reaction between \(NO_{2}(g)\) and \(CO(g)\) is represented above. The elementary steps of a proposed reaction mechanism are represented below.
Step 1: \(2~NO_{2}(g)\rightarrow NO(g)+NO_{3}(g)\) (slow)
Step 2: \(NO_{3}(g)+CO(g)\rightarrow NO_{2}(g)+CO_{2}(g)\) (fast)
(B) Rate = \(k[NO_{2}]^2\)
(C) Rate = \(k[NO_{3}][CO]\)
(D) Rate = \(k[NO_{2}][NO_{3}][CO]\)
▶️ Answer/Explanation
1. Identify the Rate-Determining Step (RDS):
In a multi-step reaction mechanism, the overall rate of the reaction is governed by the slowest step. This is known as the rate-determining step.
2. Locate the Slow Step:
The mechanism explicitly labels Step 1 as the (slow) step.
3. Write the Rate Law from the RDS:
The rate law is written based on the reactants of the slow elementary step. The reactants in Step 1 are two molecules of \(NO_2(g)\).
Step 1: \(2~NO_{2}(g)\) \(\rightarrow NO(g)+NO_{3}(g)\)
4. Formulate the Rate Law:
For an elementary step, the reaction order matches the stoichiometry. Since there are two \(NO_2\) molecules, the reaction is second-order with respect to \(NO_2\).
Rate = \(k[NO_{2}]^2\)
(Note: The reactants of the fast step, \(CO\) and the intermediate \(NO_3\), do not appear in the final rate law because they are not part of the slow step.)
✅ Answer: (B)
Question
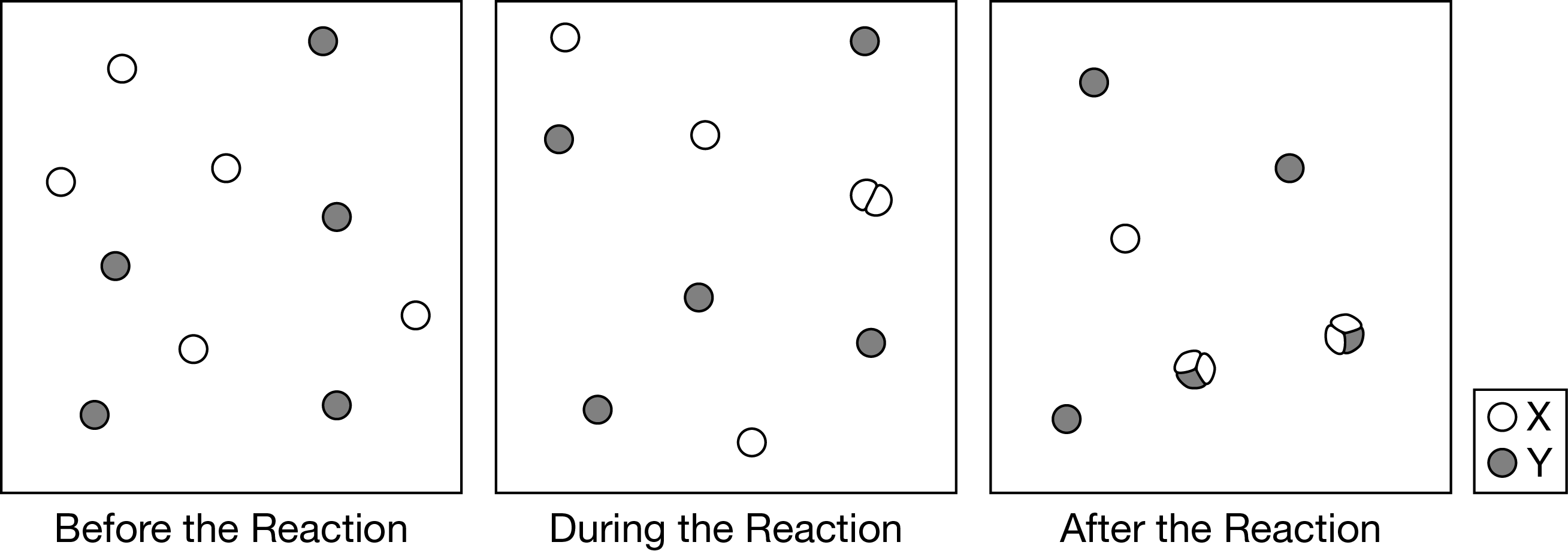
Which of the following best describes the elementary step(s) in the reaction mechanism represented in the diagram above?
A One step: \(2X(g)+Y(g)→X_2Y(g)\)
B One step: \(X_2(g)+Y(g)→X_2Y(g)\)
C Two steps: Step 1: \(X(g)+Y(g)→XY(g)\)
Step 2: \(X(g)+XY(g)→X_2Y(g)\)
D Two steps: Step 1: \(2X(g)→X_2(g)\)
Step 2: \(X_2(g)+Y(g)→X_2Y(g)\)
▶️Answer/Explanation
Ans:D
From the first diagram to the second diagram, two X atoms joined to form the intermediate \(X_2\). From the second diagram to the third diagram, the \(X_2\) joined with a Y to form the final product \(X_2Y\).
Question
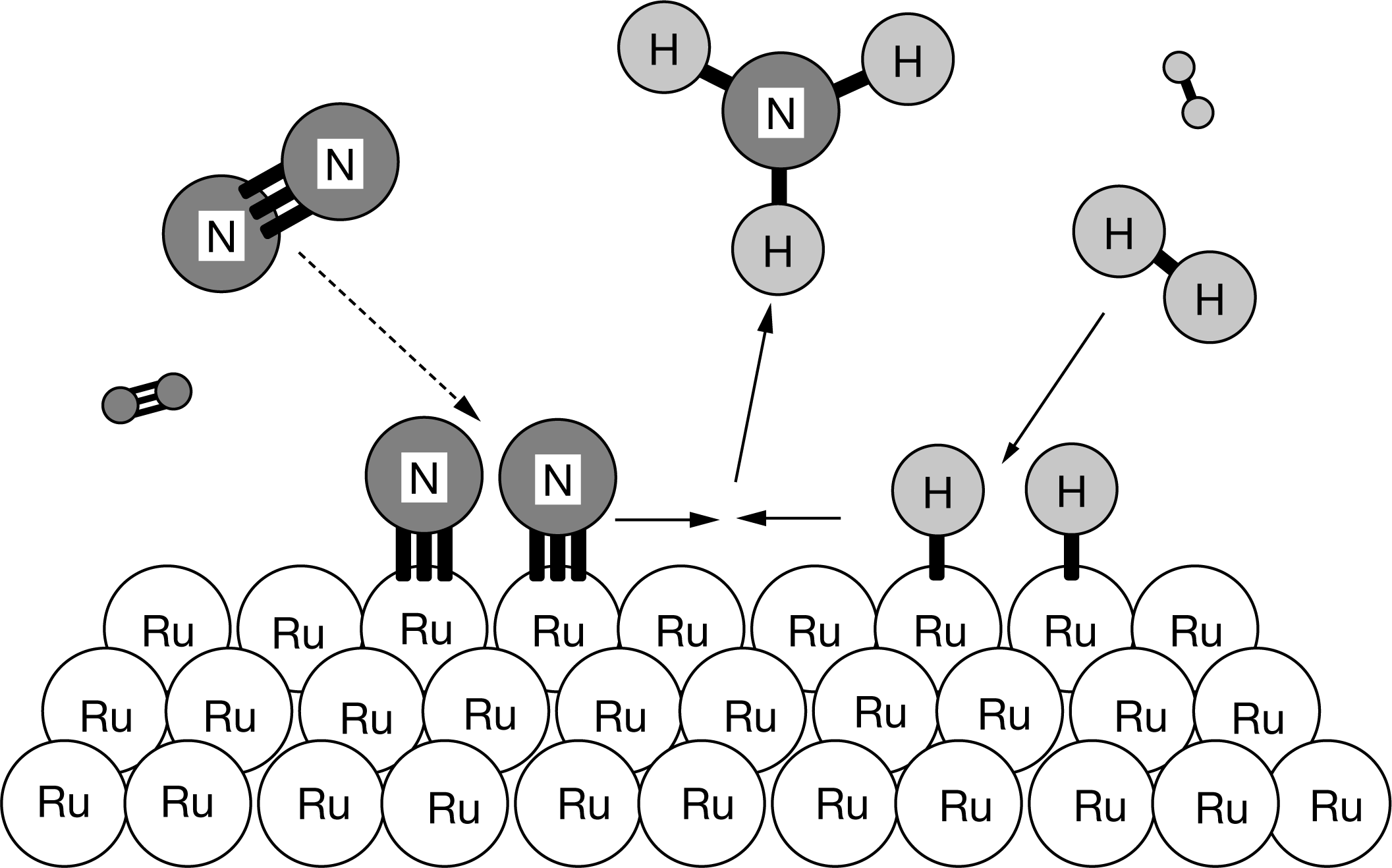
The diagram above shows the progress of the chemical reaction for the synthesis of ammonia from its elements. The adsorption of the N2 molecules on the surface of Ru weakens the triple bond between the two N atoms. Based on the diagram, what is the role of Ru
in this process?
A Ru is a catalyst.
B Ru is a reactant.
C Ru is a product.
D Ru is an intermediate.
▶️Answer/Explanation
Ans:A
The amount of Ru atoms does not change. Ru is a catalyst that provides an alternate path to the process of breaking the triple bond between the two N atoms, which is the rate-limiting step in the synthesis of \(NH_3\).
