AP Chemistry 7.2 Direction of Reversible Reactions -MCQs - Exam Style Questions
Question
colorless brown
\(K_{p}=3.0\) at \(70^{\circ}C\)
A mixture of \(NO_{2}(g)\) and \(N_{2}O_{4}(g)\) is placed in a glass tube and allowed to reach equilibrium at \(70^{\circ}C\), as represented above.
Which of the following statements best helps to explain why the contents of the tube containing the equilibrium mixture turned a lighter color when the tube was placed into an ice bath?
(B) The forward reaction is endothermic.
(C) The ice bath lowered the activation energy.
(D) The ice bath raised the activation energy.
▶️ Answer/Explanation
1. Analyze the Observation:
The mixture is \(N_{2}O_{4}(\text{colorless})\iff2~NO_{2}(\text{brown})\). The tube turning a “lighter” color means the concentration of brown \(NO_2\) gas decreased.
2. Relate to Equilibrium Shift:
A decrease in the product (\(NO_2\)) means the equilibrium shifted to the left (toward the reactant, \(N_2O_4\)).
3. Analyze the Stress:
The tube was placed in an “ice bath,” which is a decrease in temperature.
4. Apply Le Chatelier’s Principle:
Le Chatelier’s principle states that if a system at equilibrium is subjected to a change in temperature, it will shift in the direction that counteracts the change. A decrease in temperature will favor the exothermic (heat-releasing) direction.
5. Combine and Conclude:
- The stress (cooling) caused the equilibrium to shift left.
- The principle states that cooling favors the exothermic direction.
- Therefore, the reverse reaction (\(2~NO_{2} \rightarrow N_{2}O_{4}\)) must be exothermic.
- If the reverse reaction is exothermic, the forward reaction (\(N_{2}O_{4} \rightarrow 2~NO_{2}\)) must be endothermic (heat-absorbing).
✅ Answer: (B)
Questions
\(2FeO(s)\rightleftharpoons 2Fe(s)+O_{2} K_{eq}\)= \(1\times 10^{-6}\) at 1000K
\(CO_{2}(g)\rightleftharpoons C(s)+O_{2}(G) K_{eq}\)= \(1\times 10^{-32} \)at 1000K
The formation of Fe(s) and\( O_2\)(g) from FeO(s) is not thermodynamically favorable at room temperature. In an effort to make the process favorable, C(s) is added to the FeO(s) at elevated temperatures. Based on the information above, which of the following gives the value of K eq and the sign of \(\Delta G^{\circ}\) for the reaction represented by the equation below at 1000 K?
$2FeO(s)+ C_{s} \rightleftharpoons 2Fe(s)+CO_{2}$
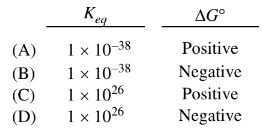
▶️Answer/Explanation
Ans: D
Given:
1. \(K_{eq}\) for \(2FeO(s) \rightleftharpoons 2Fe(s) + O_{2}\) is \(1 \times 10^{-6}\) at 1000 K.
2. \(K_{eq}\) for \(CO_{2}(g) \rightleftharpoons C(s) + O_{2}(g)\) is \(1 \times 10^{-32}\) at 1000 K.
To find the equilibrium constant \(K_{eq}\) for the overall reaction:
\[ 2FeO(s) + C_{s} \rightleftharpoons 2Fe(s) + CO_{2} \]
We need to consider the equilibrium constant for the individual reactions and apply them to determine the equilibrium constant for the overall reaction.
Given the equilibrium constants for the individual reactions, we know:
The reverse reactions are favored at 1000 K for both reactions because their equilibrium constants are very small.
This suggests that the forward reactions are thermodynamically unfavorable.
For the overall reaction, it involves the combination of two unfavorable forward reactions. Thus, the overall equilibrium constant will be extremely small, as it is the product of the equilibrium constants of the
individual reactions.
\[K_{eq}=K_{1} \times K_{2} = (1 times 10^{-6}) \times (1 \times 10^{32}) =1 \times 10^{26} \]
Given that the overall reaction proceeds in the thermodynamically unfavorable direction, the sign of \(AG^{\circ}\) for the overall reaction will be negative.
Therefore, the correct answer is:
(D) \(1 \times 10^{26}\) & Negative
Questions
\(2FeO(s)\rightleftharpoons 2Fe(s)+O_{2} K_{eq}\)= \(1\times 10^{-6}\) at 1000K
\(CO_{2}(g)\rightleftharpoons C(s)+O_{2}(G) K_{eq}\)= \(1\times 10^{-32} \)at 1000K
The formation of Fe(s) and\( O_2\)(g) from FeO(s) is not thermodynamically favorable at room temperature. In an effort to make the process favorable, C(s) is added to the FeO(s) at elevated temperatures. Based on the information above, which of the following gives the value of K eq and the sign of \(\Delta G^{\circ}\) for the reaction represented by the equation below at 1000 K?
$2FeO(s)+ C_{s} \rightleftharpoons 2Fe(s)+CO_{2}$
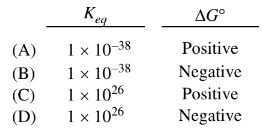
▶️Answer/Explanation
Ans: D
Given:
1. \(K_{eq}\) for \(2FeO(s) \rightleftharpoons 2Fe(s) + O_{2}\) is \(1 \times 10^{-6}\) at 1000 K.
2. \(K_{eq}\) for \(CO_{2}(g) \rightleftharpoons C(s) + O_{2}(g)\) is \(1 \times 10^{-32}\) at 1000 K.
To find the equilibrium constant \(K_{eq}\) for the overall reaction:
\[ 2FeO(s) + C_{s} \rightleftharpoons 2Fe(s) + CO_{2} \]
We need to consider the equilibrium constant for the individual reactions and apply them to determine the equilibrium constant for the overall reaction.
Given the equilibrium constants for the individual reactions, we know:
The reverse reactions are favored at 1000 K for both reactions because their equilibrium constants are very small.
This suggests that the forward reactions are thermodynamically unfavorable.
For the overall reaction, it involves the combination of two unfavorable forward reactions. Thus, the overall equilibrium constant will be extremely small, as it is the product of the equilibrium constants of the
individual reactions.
\[K_{eq}=K_{1} \times K_{2} = (1 times 10^{-6}) \times (1 \times 10^{32}) =1 \times 10^{26} \]
Given that the overall reaction proceeds in the thermodynamically unfavorable direction, the sign of \(AG^{\circ}\) for the overall reaction will be negative.
Therefore, the correct answer is:
(D) \(1 \times 10^{26}\) & Negative
